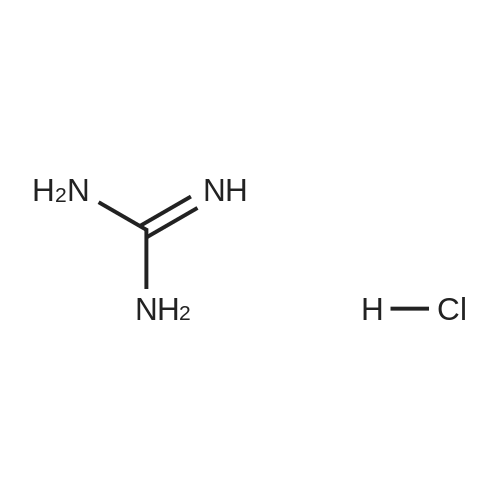
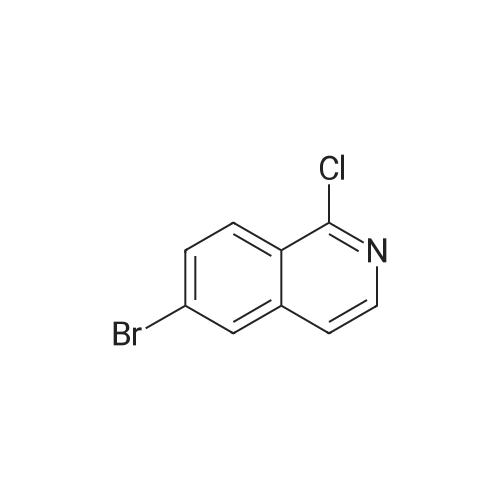
| 72% |
With trichlorophosphate; In chloroform; at 0 - 20℃; for 1.5h;Heating / reflux; |
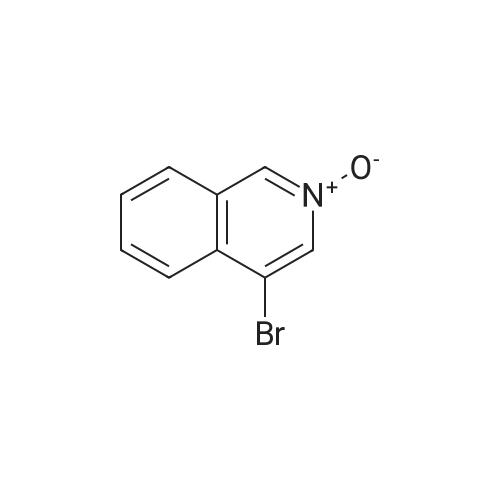
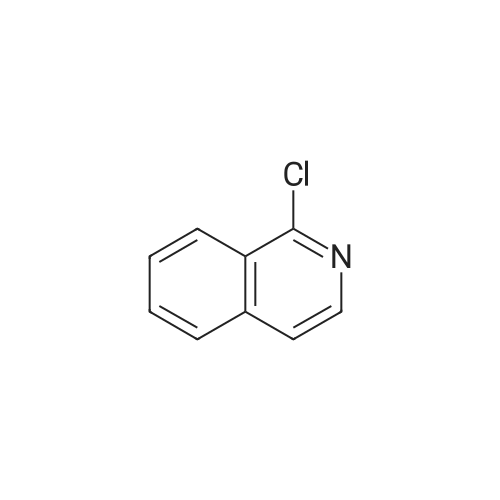
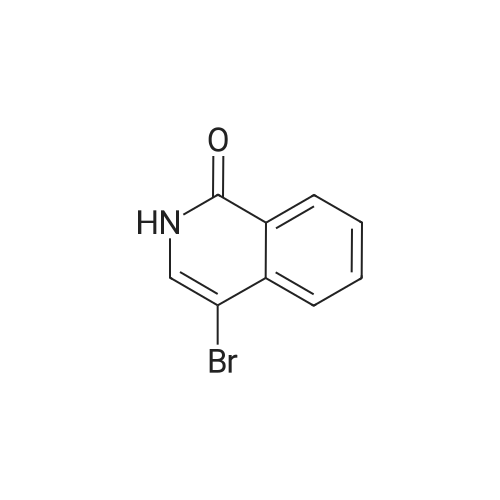
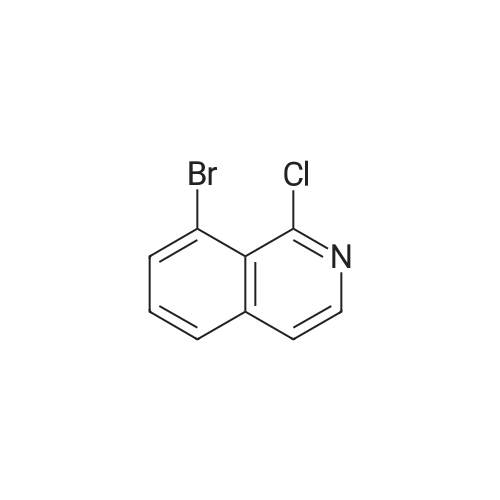
2d) 4-BROMO-1-CHLORO-ISOQUINOLINE To a solution of 4-bromoisoquinoline (52. 08 g, 0. 250 MOL) in methylene chloride (600 mL) is added m-chloroperbenzoic acid (64.47 g, 0.250 MOL). The mixture is stirred for 2.5 hours. To the mixture is added 1.5 g of m-chloroperbenzoic acid and the mixture is stirred for 30 minutes. The solution is washed with 1 N NAOH, brine, and then dried over sodium sulfate. The solvent is removed to give a white solid. The solid is crystallized from hot acetone to yield 32.22 g (57.6%) of a white SOLID. 1H, 13C NMR consistent with structure. The N-oxide (15.75 g, 0.0703 mol) is dissolved in chloroform (50 mL) and cooled in an ice bath. Phosphorus OXYCHLORIDE (20 mL) is added dropwise and then the mixure is warmed to room temperature and then heated to reflux for 1.5 hours. The mixture is allowed to cool to room temperature and is then poured over ice. The aqueous mixture is neutralized to pH 7-8 with NAHCO3 AND then extracted with chloroform. The organic phase is washed with brine, dried over sodium sulfate and the solvent is removed. The residue is purified by flash chromatography (SIO2/5% ETHYL acetate/hexanes). Collected 12.22 g (72%). M+H = 389. 'H NMR ; 5 8. 50 (s, 1H), 8.40 (d, 1H), 8.20 (d, 1H), 7.92 (t, 1H), 7.79 (t, 1H). |
| 52% |
With trichlorophosphate; In dichloromethane; N,N-dimethyl-formamide; at 0 - 25℃;Inert atmosphere; |
General procedure: To a stirred solution of the appropriate azine N-oxides in anhydrous CH2Cl2 (0.1M) at 0 C is added POCl3 (1.2 equiv) followed by dropwise addition of DMF (0.5 equiv) under argon. The resulting reaction mixture was warmed to 25 C and stirred for several hours until the reaction is complete as indicated by TLC. Saturated aqueous sodium carbonate solution is added to the reaction mixture slowly to adjust the pH to 7~8. The resulting mixture is separated and the aqueous phase is extracted with CH2Cl2 thoroughly. The organic phase is combined and washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure to afford the crude product, which is purified by flash column chromatography using PE/EA (80:1) as eluent. |
|
With sodium hydrogencarbonate; trichlorophosphate; In dichloromethane; 1,2-dichloro-ethane; |
(b) A yellow suspension of 4-bromoisoquinoline N-oxide, P-1a, (6.9 g, 30.8 mmol. 1.0 eq) in 1,2-dichloroethane (60 mL) was treated with phosphorus oxychloride (Aldrich, 9.0 mL, 96.4 mmol, 1.8 eq) and warmed to 80 C. After 1.5 hours, the resultant green suspension was carefully poured into a cold solution of 50% saturated sodium bicarbonate (500 mL) and the aqueous layer was extracted with diethyl ether (3*300 mL). The combined organic extracts were washed with water (200 mL), brine (200 mL), dried over magnesium sulfate, and concentrated under reduced pressure to give a tan solid (6.8 g). The crude product was dissolved in a minimal amount of dichloromethane and purified by flash chromatography over silica gel using 5% ether/cyclohexane to give 4-bromo-1-chloro-isoquinoline, P-1b, as a white solid (5.7 g, 77%): HPLC Rf=15.4 min.; TLC Rf=0.4 (5% ether/cyclohexane); 1H NMR (300 MHz, DMSO-d6) delta8.68 (s, 1H), 8.42 (d, 1H, J=8.4 Hz), 8.26 (d, 1H, J=8.3 Hz), 8.15 (t, 1H, J=7.6 Hz), 8.02 (t, 1H, J=7.6 Hz); 13C NMR (75 MHz, DMSO-d6) delta150.4, 143.0, 135.8, 133.8, 130.8, 127.3, 126.8, 126.5, 119.0; MS (ESI) m/z 242/244 [M+H]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping