| 73% |
|
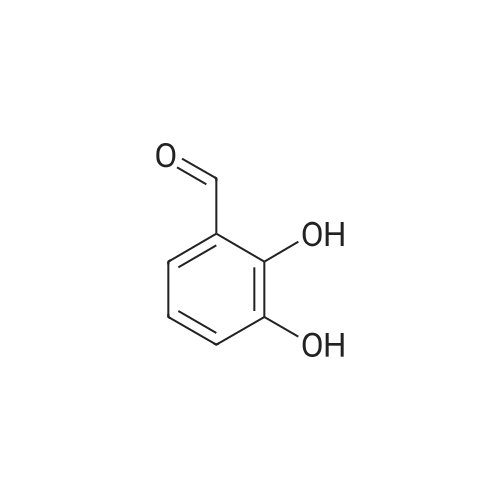
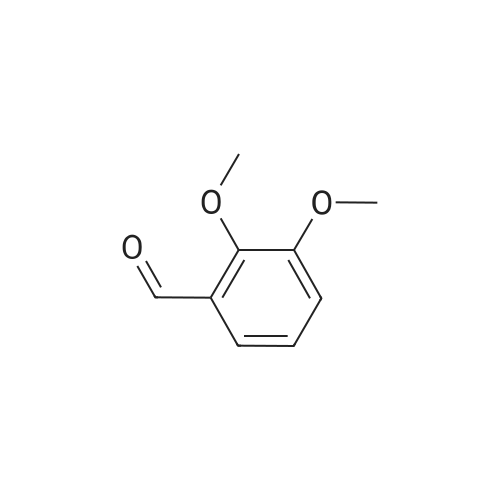
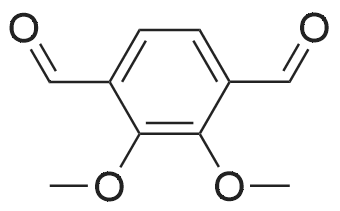
To a stirred solution of 2,3-dihydroxybenzaldehyde (1.00 g, 7.24 mmol) in anhydrous DMF (20 mL) was added K2CO3 (1.00 g, 7.24 mmol) and the mixture was stirred at room temperature for 30 minutes. CH3I (0.54 mL, 8.68 mmol) was then slowly added to the mixture and stirred for 8 hours.After completion of the reaction, it was neutralized with 1N HCl and extracted with ether (3 x 30 mL).The combined organic solvent layers were washed with water (3 x 40 mL), brine (3 x 40 mL), dried over anhydrous Na2SO4 and concentrated in vacuo.The crude compound was purified by column chromatography (EtOAc: Hexane = 1: 4) to give a white solid compound 10 (0.80 g, 73%). |
| 62% |
|
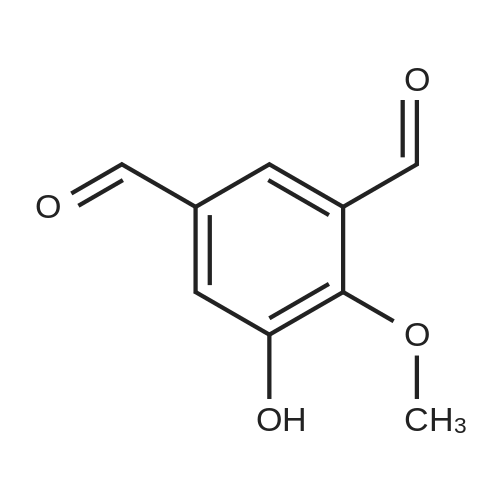
A solution of 2, 3-dihydroxybenzaldehyde (10 g, 72 [MMOL)] in anhydrous DMF (100 mL) was treated with K2CO3 (10 g, 72 [MMOL)] at [25C] and the mixture was stirred for 30 minutes. [LODOMETHANE] (4.9 mL, 80 [MMOL)] was added and the reaction was further stirred for 20 h. The reaction was quenched with water and extracted with diethyl ether. The organic layer was dried using sodium sulfate and solvents were evaporated under vacuum. The residue was purified by Biotage to obtain 3-hydroxy-2- [METHOXYBENZALDEHYDE] (6.8 g, 45 mmol, 62%) as a white [SOLID.'H] NMR [(CDC ! S) 5] 4.0 (s, 3H), 5.9 (s, [1H),] 7.2 (m, 2H), 7.4 (m, 1H), 10.3 (s, [1H)] ; GC/MS (ES) 153 [(M+1) +.] |
| 57% |
|
A solution of 2,3-dihydroxybenzaldehyde (1.0 g, 7.24 mmol) in DMF (10 mL) was treated with K2CO3 (1.0 g, 7.24 mmol) and the mixture was stirred at rt for 30 min. Iodomethane (0.50 ml, 7.96 mmol) was added and the reaction was further stirred for 20 h. The reaction was quenched with H2O and extracted with Et2O. The organic layer was dried over Na2SO4 and concentrated. The residue was purified flash column chromatography to afford the title compound (0.63 g, 57%) as a colorless needle.1H NMR δ (CDCl3) 10.28 (1H, s), 7.39 (1H, dd, J=7.8 Hz, 1.8 Hz), 7.25 (1H, dd, J=7.8 Hz, 1.8 Hz), 7.17 (1H, t, J=7.8 Hz), 5.81 (1H, s), 3.99 (1H, s)ESI-MS [M+H]+: 153 |
| 45% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 18h; |
General procedure: The compound of Formula (VI) is synthesized as shown in the following scheme: |
| 40% |
With potassium carbonate; In N,N-dimethyl-formamide; at 20℃; for 20h; |
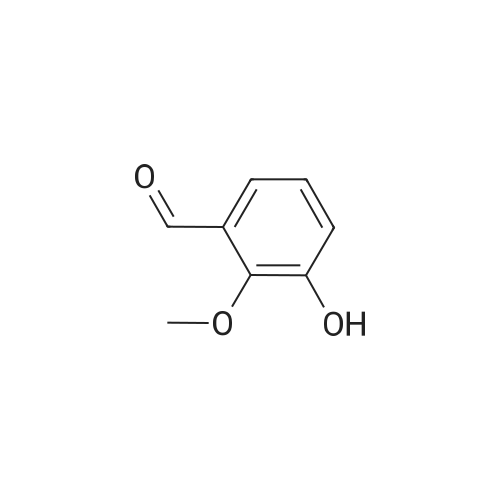
Reference Example 52 3-hydroxy-2-methoxybenzaldehyde; [Show Image] To a solution (80 mL) of 2,3-dihydroxybenzaldehyde (5.00 g) in N,N-dimethylformamide were added potassium carbonate (4.93 g) and a solution (20 mL) of iodomethane (6.89 g) in N,N-dimethylformamide, and the mixture was stirred at room temperature for 20 hr. Water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane-ethyl acetate=100:0→60:40) to give the title compound as a white solid (yield: 2.16 g, 40%). 1H-NMR (CDCl3, 300 MHz):δ3.98 (3H, s), 5.83 (1H, s), 7.15 (1H, dd, J = 8 . 1, 7.7 Hz), 7 . 24 (1H, dd, J = 8.1, 1.8 Hz), 7.38 (1H, dd, J = 7.6, 1.8 Hz), 10.27 (1H, s). |
|
|
[0308] 2,3-dihydroxybenzaldehyde (27.6 g) (200 mmol) is mixed with dry DMF (460 mL) and KHCO3 (80 g, 800 mmol), under N2 atmosphere. The mixture is stirred at room temperature for 30 min. MeI (51 mL, 820 mmol) is added in one portion. The mixture is further stirred at room temperature for 30 h. The excess MeI is evaporated in vacuo. H2O (1.1 L) is added, followed by 37% HCl (46 mL) to reach pH ~3.5 after the addition. The mixture is extracted with Et2O (4x0.55 L then 1x0.28 L). The combined organic layer is further washed with saturated NH4Cl (2x0.35 L), then brine (1x0.7 L), dried on MgSO4, filtered and evaporated in vacuo to yield the crude desired product. This crude is dissolved in Et2O (0.7 L), and extracted with 1M NaOH (2x0.42 L). The combined aqueous layer is treated with 37% HCl (71 mL) to reach pH ~ 1. The resulting suspension is cooled to 15C, filtered on a Buchner filter, the solid is washed with H2O (2x30 mL), dried under suction and then in vacuo at 42C to yield the desired product. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping