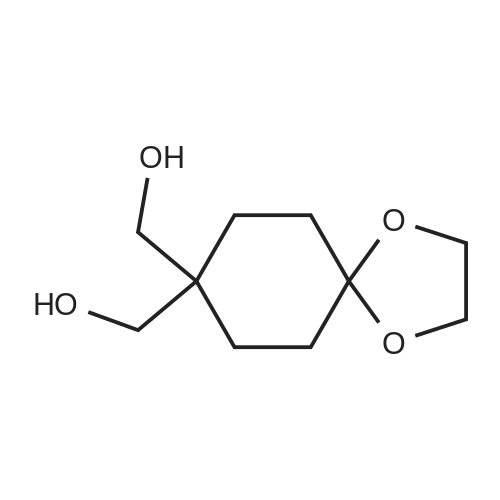
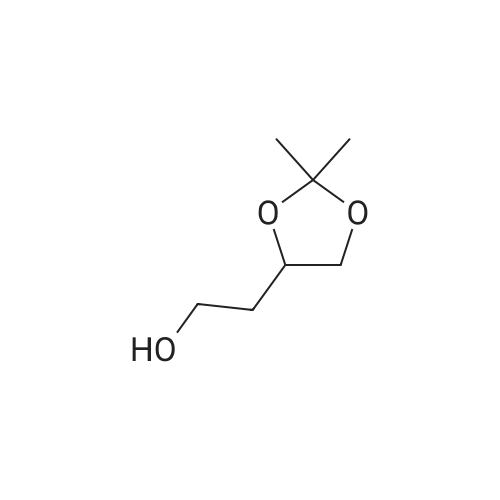
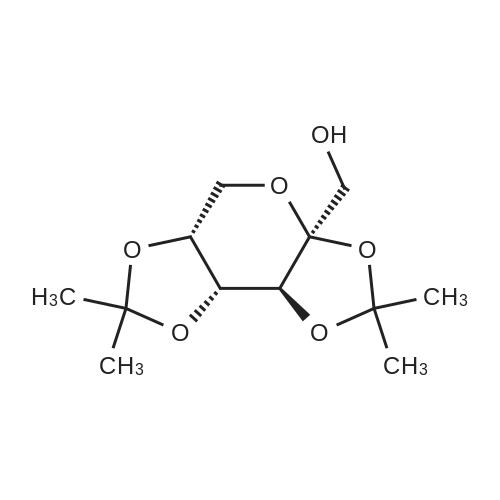
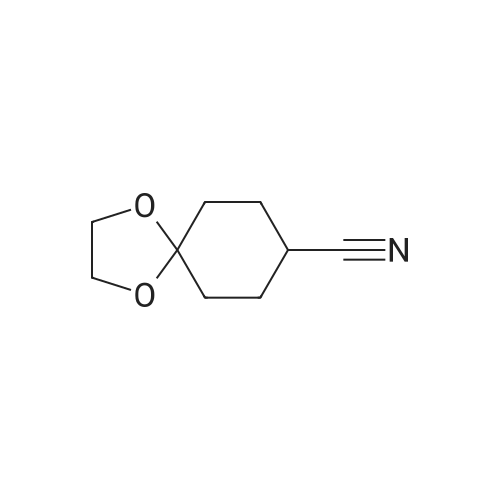
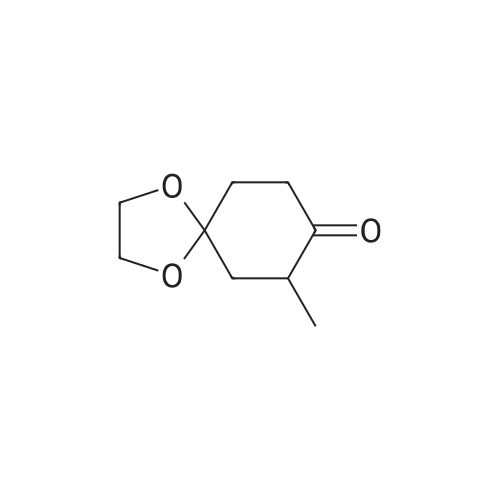
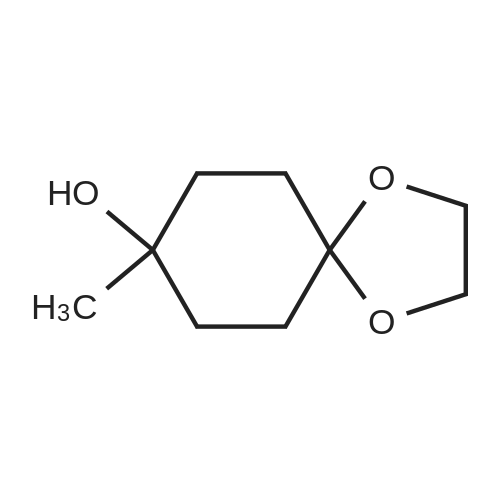
| 86.7% |
With hydrogenchloride; In tetrahydrofuran; water; at 20℃; |
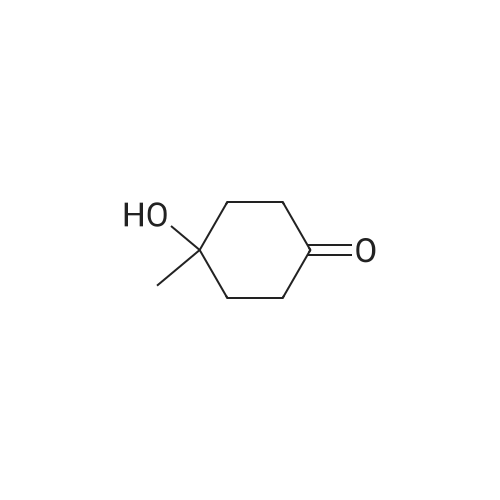
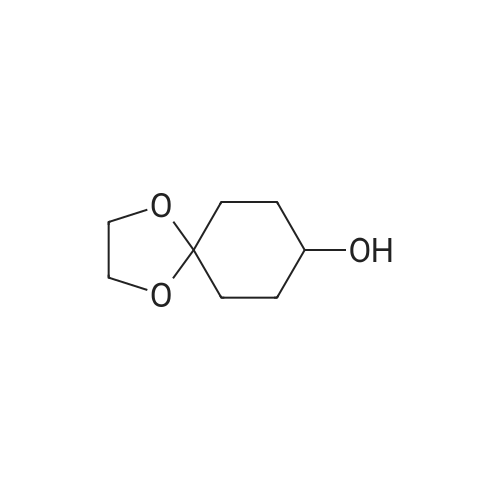
Step 2: synthesis of 4-hydroxy-4-methylcyclohexanone To a solution of 8-methyl-1,4-dioxaspiro[4.5]decan-8-ol (6.1 g, 35.5 mmol) in THF (200 mL) was added 2 N HCl (32 mL). The resulting mixture was stirred at RT overnight, and then was basified to pH 8.0 by saturated K2CO3 solution. The separated organic layer was concentrated in vacuo and the residue was purified by column chromatography on silica gel (EA:PE=3:20 to 2:3) to afford the title compound as yellow oil (4.1 g, yield: 86.7%). |
| 86.7% |
With hydrogenchloride; In tetrahydrofuran; water; at 20℃; |
To a solution of 8-methyl-1,4-dioxaspiro[4.5]decan-8-ol (6.1 g, 35.5 mmol) in THF (200 mL) was added 2 N HCl (32 mL). The resulting mixture was stirred at RT overnight, and then was basified to pH 8.0 by saturated K2CO3 solution. The separated organic layer was concentrated in vacuo and the residue was purified by column chromatography on silica gel (EA:PE=3:20 to 2:3) to afford the title compound as yellow oil (4.1 g, yield: 86.7%). |
| 84% |
With hydrogenchloride; water; In acetone; at 40℃; for 14h;Inert atmosphere; |
Step 2. Preparation of 4-hydroxy-4-methylcyclohexanone To a solution of 8-methyl-l,4-dioxaspiro[4.5]decan-8-ol (5.26 g, 30.5 mmol) in acetone (40 mL) and water (60 mL) at room temperature was added 4 M HC1 (22.91 mL, 92 mmol). The reaction mixture was heated at 40 C for 14 h. The mixture was cooled to room temperature and was neutralized by the addition of solid sodium carbonate. The acetone was removed on the rotovapor and the aqueous layer was extracted with ethyl acetate (7 x 150 mL). The combined organic layers were dried over MgS04, filtered, and concentrated. The product was purified by column chromatography on silica gel (70% ethyl acetate in hexanes) to afford 4-hydroxy-4-methylcyclohexanone (3.27 g, 25.5 mmol, 84% yield) as a white solid: 1H NMR (400MHz, CHLOROFORM-d) delta 2.80 - 2.68 (m, 2H), 2.30 - 2.20 (m, 2H), 2.04 - 1.94 (m, 2H), 1.92 - 1.80 (m, 2H), 1.56 (s, 1H), 1.39 (s, 3H); 13C MR (100MHz, CHLOROFORM-d) delta 21 1.55, 68.14, 38.40, 36.80, 29.51. |
| 82% |
With hydrogenchloride; In tetrahydrofuran; water; at 20℃; for 16h; |
Compound 1A (5.1 g, 29.6 mmol) was dissolved in THF (100 mL), followed by addition of 1N aqueous HCl (44.4 mL, 44.4 mmol) at room temperature. The resulting mixture was stirred at room temperature for 16 h. The resulting reaction liquid was concentrated under reduced pressure and then extracted with 10% MeOH/DCM (2*200 mL). The combined organic layer were washed with brine (50 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford 1B (yellow liquid, 3.1 g, 24.19 mmol, 82% yield) which was used in next step without further purification. 1H NMR (300 MHz, CDCl3) delta 2.81-2.65 (m, 2H), 2.32-2.15 (m, 2H), 2.01-1.75 (m, 4H), 1.36 (s, 3H). |
|
With hydrogenchloride; water; at 20℃; for 48h; |
A mixture of 8-methyl-1 ,4-dioxa-spiro[4.5]decan-8-o. (Step BB.7, 9.35 g, 54.3 mmol), water (400 ml) and hydrochloric acid (0.3 ml) was stirred at room temperature for 48 hours and then extracted 3X with ethyl acetate. The combined organic layers were dried over magnesium sulphate and evaporated at 40 C under a vacuum of 170 mbar to give the title compound as a pale yellow oil. 1H NMR (400 MHz, CDCI3) delta ppm 1.29-1.40 (m, 1 H), 1.58 (s, 1 H), 1.85 (td, 1 H), 1.97 (qd, 1 H), 2.24 (d, J = 14.8 Hz, 1 H), 2.72 (td, J = 13.7, 6.3 Hz, 1 H). |
|
With hydrogenchloride; In tetrahydrofuran; water; at 20℃; |
To a solution of 8-methyl-1 ,4-dioxaspiro[4.5]decan-8-ol (1 1 .78 g, 68.4 mmol) in THF (50 mL) was added aqueous HCI (1 M, 205 mL, 205.2 mmol). The reaction mixture was allowed to stir overnight at room temperature. Then, a saturated solution of Na2C03 was added and the mixture extracted with DCM. The combined organic extracts were dried over Na2S04, filtered and concentrated under reduced pressure to afford crude 4-hydroxy-4-methyl-cyclohexanone (6.18 g, 48.2 mmol, 71 % yield). NMR (400 MHz, CDCI3, delta): 2.70-2.61 (m, 2H), 2.21 -2.14 (m, 2H), 1 .94-1 .87 (m, 2H), 1 .83-1 .75 (m, 2H), 1 .31 (s, 3H), 1 .23 (s, 1 H). |
|
With hydrogenchloride; In tetrahydrofuran; at 0 - 20℃; for 14h; |
General procedure: Hydrochloric acid (1 M, 3.0 eq.) was added to a suspension of 1 ,3-dioxolane derivative (1.0 eq.) in THF (1 M), cooled at 0 C. The reaction mixture was allowed to return to RT and stirred for 14 h. The mixture was then carefully basified with a saturated solution of sodium carbonate and the aqueous layer was extracted with DCM (x 3). The combined organic extracts were filtered over a phase separator and concentrated under reduced pressure to afford crude carbonyl; Following general procedure J, 8-methyl-i ,4-dioxaspiro[4.5]decan-8-ol (2.50 mmol) in THF (2.5 mL)afforded the titled compound crude (2.50 mmol) as a brown oil. 1H NMR (400 MHz, CDCI3, ): 2.71 -2.59(m, 2H), 2.23-2.12 (m, 2H), 1.96-i .86 (m, 2H), 1.85-i .73 (m, 2H), 1.31 (5, 3H), 1.23 (5, 1H) |
|
With hydrogenchloride; In water; at 70℃; for 2.5h; |
To a stirred solution of 0.05 N HCl (1800 mL) was added 8-methyl-1,4- dioxaspiro [4.5] decan-8-ol (140.0 g, 0.814 mol). The mixture was stirred at 70 C for 2.5 hours. The resulting mixture was cooled to room temperature and added NaCl solid to saturation, then extracted with EtOAc (5 700 mL). The combined organic phase was dried over Na 2SO 4 and concentrated to give the 4-hydroxy-4-methylcyclohexan-1-one (105.0 g, crude) as a yellow oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping