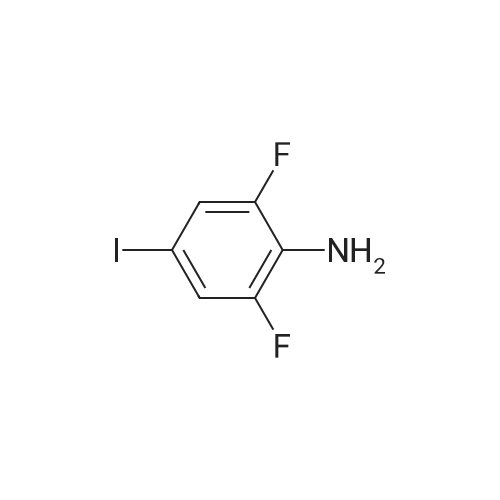
| 91% |
With tin(II) chloride dihdyrate; In ethanol; for 1.5h;Reflux; |
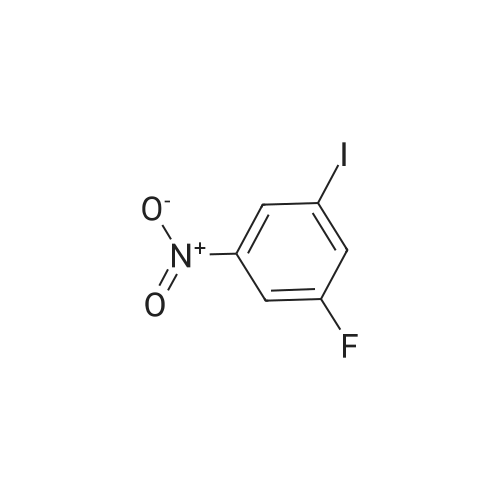
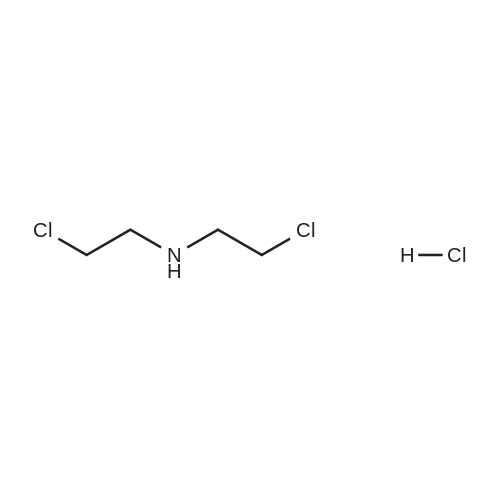
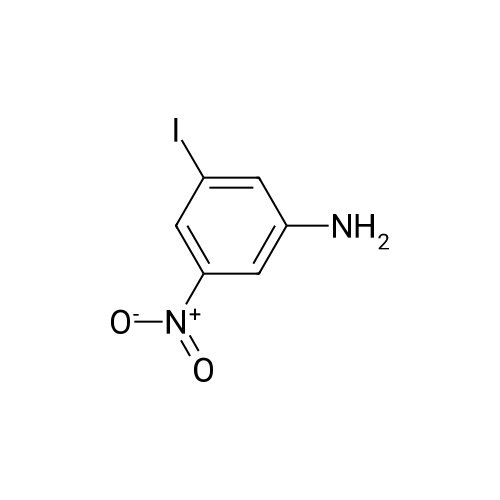
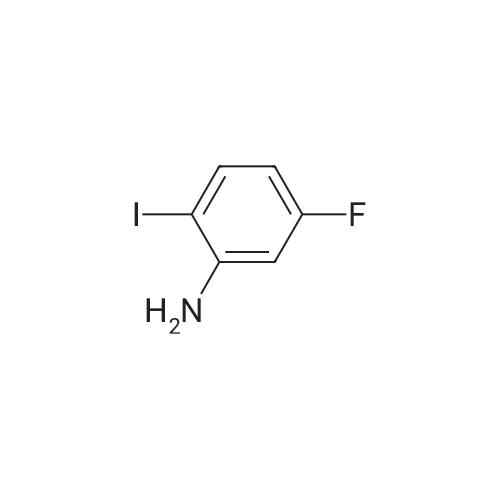
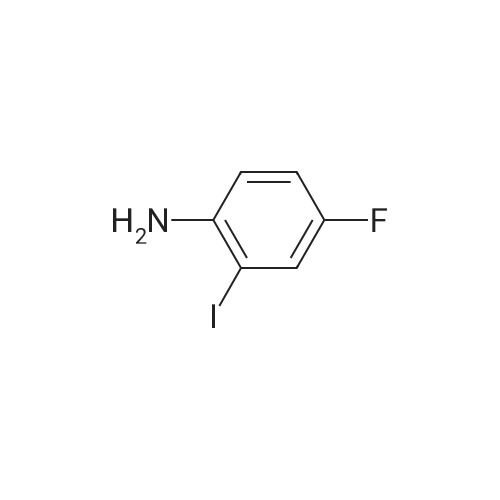
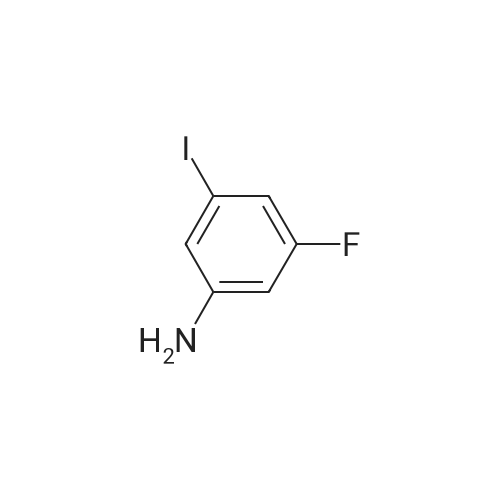
Example 2Synthesis of 3-fluoro-5-iodo-N,N-dimethylaniline (VMY-2-119): The suspension of l-fluoro-3-iodo-5 -nitrobenzene (0.5 g, 1.87 mmol) and SnCl2'2H20 (1.5 g, 6.64 mmol) in EtOH (10 mL) was heated to reflux for 1.5 h. The solvent was removed and the crude mixture was diluted with ether, washed with 4 N NaOH and brine. The ether layer was- 42 -B4067289v2 separated and dried over Na2S04, filtered, concentrated to yield amine compound as a solid (0.4 g, 91%). The crude product was used without purification.A solution of above amine (0.4 g, 1.69 mmol) and iodomethane (0.719 g, 5 mmol) in dimethylformamide (DMF; 10 mL) containing potassium carbonate (0.46 g, 3.38 mmol) was stirred for 48 h at room temperature. Water (lOmL) was then added and the solution was extracted with ether three times. The organic extracts washed with water, brine, dried over Na2S04, filtered and concentrated. The crude product was purified by column chromatography to yield VMY-2-119 as a liquid (0.23 g, 52%). 1H NMR (399 MHz) ? 6.69 - 6.63 (m, 2H), 6.22 (dt, J = 12.5, 2.3, 1H), 2.83 (s, 6H). 13C NMR (100 MHz) ? 163.33(d, JF-C=245 Hz), 152.49(d, J=l lHz) 116.91 (d, J = 2.4), 112.12(d, J=24 Hz), 98.73(d, J=26Hz), 94.24(d, J=l l Hz), 40.21 (s, 3H). |
|
With iron; acetic acid; In tetrahydrofuran; water; at 40℃; |
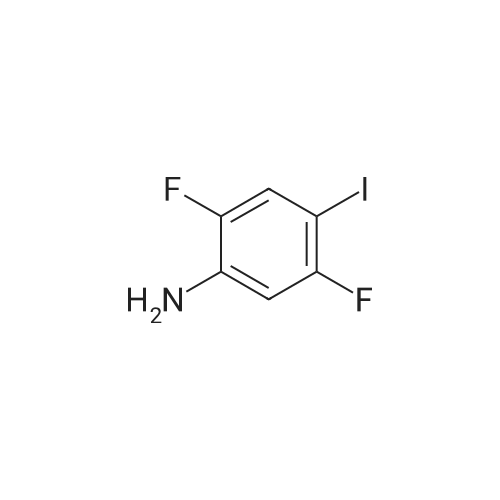
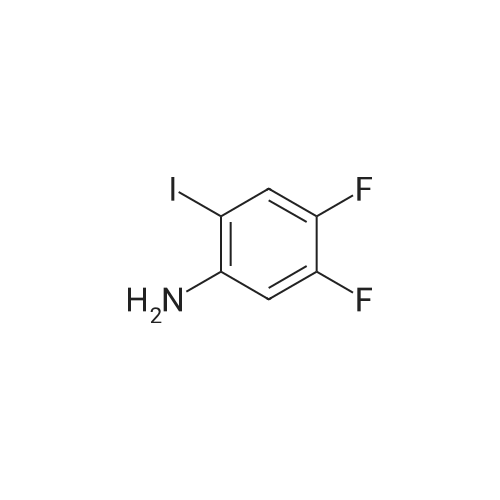
3-Iodo-5-fluoro-1-nitrobenzene (5.2 g; 19.48 mmol) is dissolved in a 4/3 water/tetrahydrofuran mixture. Powdered iron (8.7 g; 156 mmol), followed by acetic acid (4.5 ml) are added at room temperature and the suspension formed is stirred overnight at 40 C. The reaction mixture is filtered over diatomaceous earth and poured on to water. The mixture is extracted with ethyl acetate and the combined organic phases are dried over sodium sulphate, filtered and concentrated. The residue is chromatographed over silica gel with n-hexane/ethyl acetate (3/1). The pure fractions are combined and the solvent is evaporated off. The residue is diluted with methylene chloride (30 ml), and pyridine (4,0 ml; 49.5 mmol) and trifluoroacetic anhydride (3.44 ml; 24.7 mmol) are added and the mixture is stirred for one hour at room temperature. The reaction solution is poured on to aqueous hydrochloric acid (1 M; 50 ml) and the mixture is extracted twice with ethyl acetate (50 ml). The combined organic phases are dried over sodium sulphate, filtered and concentrated. The residue is chromatographed over silica gel with n-hexanelethyl acetate (9/1). The pure fractions are combined and the solvent is evaporated off. [00367] Yield: 5.40 g (98%) as a white solid. [00368] MS: 333 (M) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping