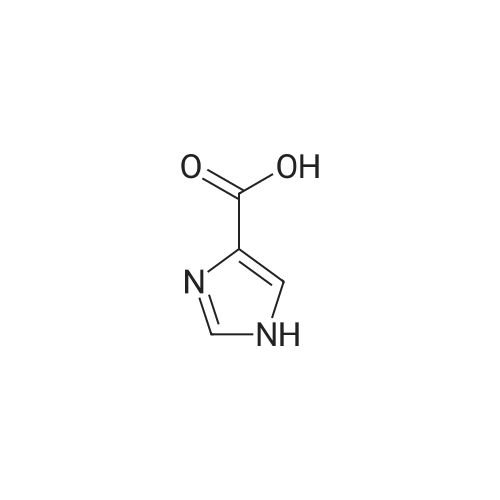
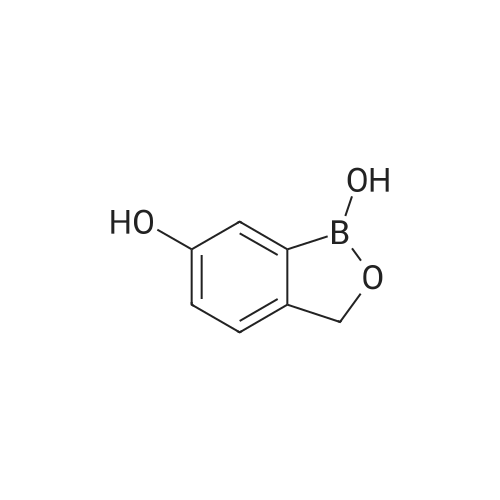
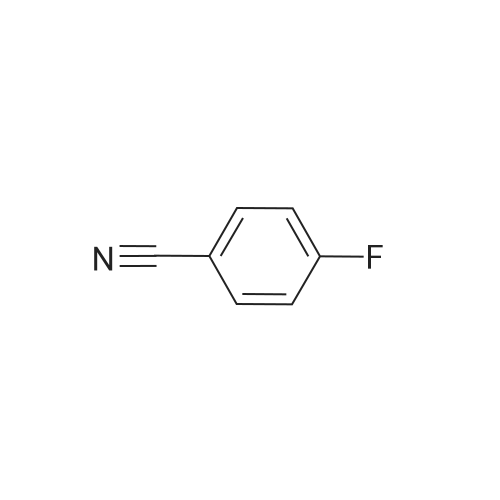
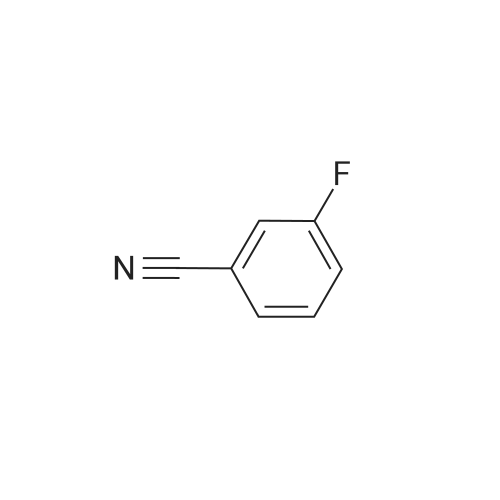
| 65.7% |
|
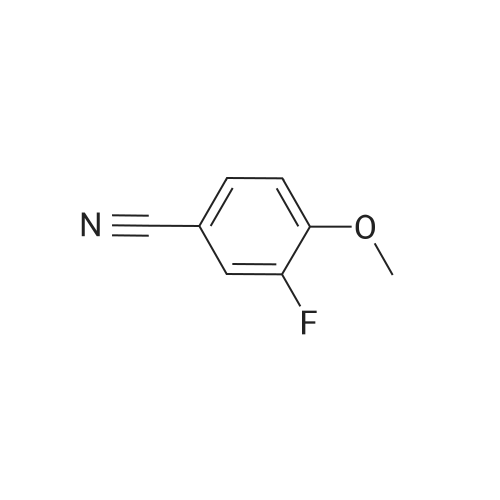
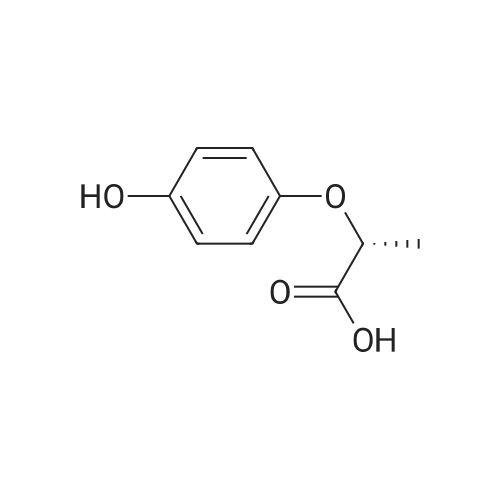
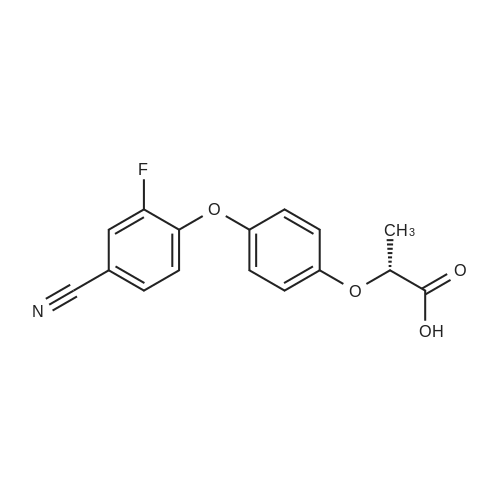
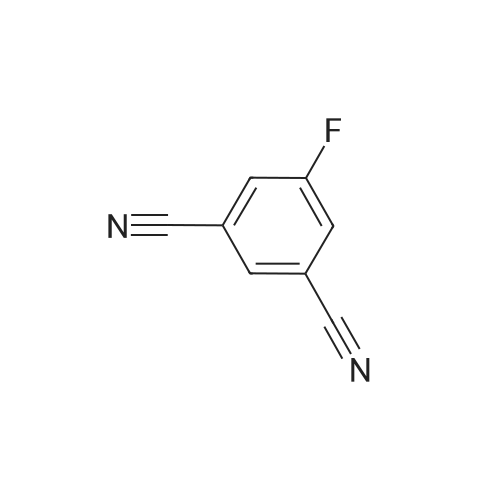
In N, N-dimethylformamide (DMF, 40 mL)Join(R) -2- (4-hydroxyphenoxy) propionic acid (3.03 g, 0.02 mol)Potassium carbonate (5.52 g, 0.04 mol) was added in portions,Heating to 70 ~ 80 , stirring for 1h,Join by volume3,4-difluorobenzonitrile (2.38 g, 0.02 mol)Continue stirring reaction 6 ~ 7h. Cooled to room temperature,Poured into ice water (250 mL)Slowly add dilute hydrochloric acid, adjusted to pH 4 ~ 5, suction filter, washed, dried in a vacuum oven(R) -2- [4- (4-cyano-2-fluorophenoxy) phenoxy] propionic acidGray solid(R) -2- [4- (4-cyano-2-fluorophenoxy) phenoxy] propionic acid3.26 g, yield 65.7%. |
| 65.7% |
|
In N, N-dimethylformamide (DMF, 40 mL)(R) -2- (4-hydroxyphenoxy) propionic acid (3.03 g, 0.02 mol) was added,Potassium carbonate (5.52 g, 0.04 mol) was added in portions,Heating to 70 C to 80 C,Continuous stirring 1h,(2.38 g, 0.02 mol) of 3,4-difluorobenzonitrile was added in portions,Continue stirring reaction 6 ~ 7h.Cooled to room temperature,Poured into ice water (250 mL)Slowly adding dilute hydrochloric acid,Adjusted to pH 4 ~ 5, suction filter,Washed,Dried in a vacuum oven(R) -2- [4- (4-cyano-2-fluorophenoxy) phenoxy] propionic acid as a gray solid(R) -2- [4- (4-cyano-2-fluorophenoxy) phenoxy] propionic acid3.26g,Yield 65.7%. |
| 65.7% |
|
In N,N-dimethylformamide (DMF, 40 mL),Add <strong>[94050-90-5](R)-2-(4-hydroxyphenoxy)propionic acid</strong> (3.03 g,0.02mol),Potassium carbonate (5.52 g, 0.04 mol) was added in portions.Warming up to 70 C ~ 80 C,Stirring for 1 hour,3,4-difluorobenzonitrile (2.38 g, 0.02 mol) was added in portions,Stirring reaction was continued for 6-7 hours.Cool to room temperature,Pour into ice water (250mL),Slowly add dilute hydrochloric acid,Adjust to pH 4 ~ 5, suction filtration, water wash,Dryed by vacuum drying oven(R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionic acidGray solid(R)-2-[4-(4-Cyano-2-fluorophenoxy)phenoxy]propanoic acid 3.26 g,The yield was 65.7%. |
|
With 1,4-diaza-bicyclo[2.2.2]octane; 18-crown-6 ether; potassium carbonate; In N,N-dimethyl acetamide; at 60℃; for 3h; |
Was charged into a 250 mL four-necked flaskN, N-dimethylacetamideN, N-dimethylacetamide100 mL,Potassium carbonate 50 g,Triethylene diamineTriethylene diamine5 g and a phase transfer catalyst18-crown-60.3 g,And then put into batches(R) -2- (4-hydroxyphenoxy) propionic acid (26 g, 0.14 mol)A large number of bubbles are generated;(R) -2- (4-hydroxyphenoxy) propionic acid was added and 3,4-difluorobenzonitrile was added20 g (0.14 mol),Then, the temperature was raised to 60 C, and the reaction was allowed to proceed for 3 hours while maintaining the reaction. The solvent was distilled off under reduced pressure, and the solution was cooled to room temperature and 150 mL of water was added theretoDissolved in 15% dilute hydrochloric acid to adjust the pH value to 4 to 5, stirring precipitation of solid, filtered(R) -2- [4- (2-fluoro-4-cyano) -phenoxy] -propionic acid |
|
With tetrabutylammomium bromide; potassium carbonate; In N,N-dimethyl-formamide; at 60 - 70℃; for 2h; |
500 g of DMF was added to a 1000 mL four-necked flask, 100 g of compound A was charged,113.6 g of potassium carbonate and 5 g of tetrabutylammonium bromide, the temperature was raised to 60 C, and 83.9 g was added dropwise3,4-difluorobenzonitrile in 50 g of DMF in a mixed solution at a controlled temperature of 65 to 70 C,After completion of the dropwise addition, the reaction was allowed to proceed for about 2 hours, the reaction was completed,With hydrochloric acid to adjust the pH to 7 ~ 8, stirring 1 hour, filtration, the solid is the etherification (compound F), drying. |
|
With tetrabutylammomium bromide; sodium carbonate; In toluene; at 55 - 60℃; for 2h; |
To a 1000 mL four-necked flask was added 500 g of toluene,100 g of compound A was charged,87.3 g of sodium carbonate and 5 g of tetrabutylammonium bromide,Heating up to 60 ,A mixed solution of 83.9 g of compound B in 50 g of toluene was added dropwise,Control temperature 60 ~ 55 ,Drop finished,Reaction for about 2 hours,The reaction ends,Added to 1000 g of water,With hydrochloric acid to adjust the pH to 7 ~ 8,Stirring for 1 hour,Layered,The etherate extract is ready for use. |
|
With tetrabutylammomium bromide; potassium carbonate; In N,N-dimethyl-formamide; at 60 - 70℃; for 2h; |
Into a 1000 mL four-necked flask, 500 g of DMF was charged and 100 g of Compound A, 1 was charged13.6 g of potassium carbonate and 5 g of tetrabutylammonium bromide, warmed to 60 C.,A mixed solution of 83.9 g of compound B in 50 g of DMF was added dropwise thereto at a controlled temperature of 65 to 70 C,The addition was complete, the reaction for about 2 hours, the reaction was completed, added to 1000g of water,With hydrochloric acid to adjust the pH to 7 ~ 8, stirred for 1 hour, filtered,The solid is etherified, dried. |
|
With 1,4-diaza-bicyclo[2.2.2]octane; 18-crown-6 ether; potassium carbonate; In N,N-dimethyl acetamide; at 60℃; for 3h; |
To the 250 ml four-mouth flask, input N,N-dimethylacetamide 100 ml, potassium carbonate 50 g, triethylenediamine 5 g and a phase transfer catalyst 18-crown-6 0.3g. Then by batches input <strong>[94050-90-5](R)-2-(4-hydroxyphenoxy)propionic acid</strong> 26 g (0.14 mol), a large number of bubble generated; Wait until <strong>[94050-90-5](R)-2-(4-hydroxyphenoxy)propionic acid</strong> inputting is finished, and then input 3,4-difluorobenzonitrile 20 g (0.14 mol), then heat to 60 C, maintain temperature reaction 3 hours. End of the reaction. The solvent is distilled under reduced pressure steam in addition to, the room temperature water 150 ml dissolved, for 15% dilute hydrochloric acid to adjust the pH value to 4-5, stirring precipitated solid, filtered to obtain the (R)-2-[4-(2-fluoro-4-cyanophenoxy)phenoxy]propanoic acid and set aside. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping