|
With triethylamine;aluminum nickel; In ethanol; |
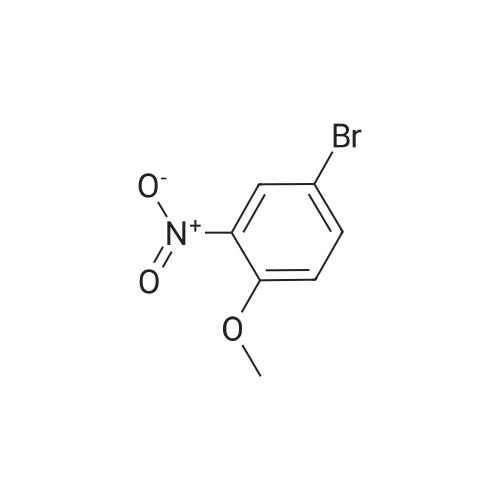
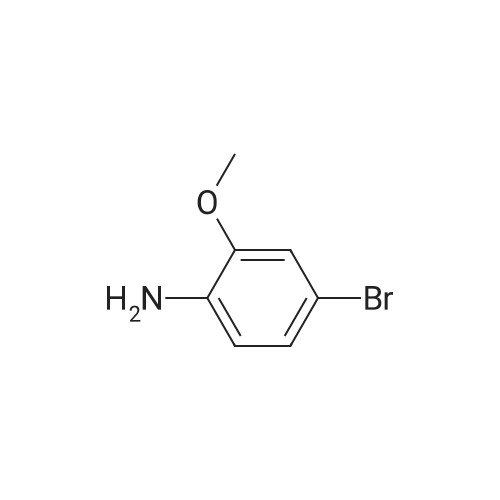
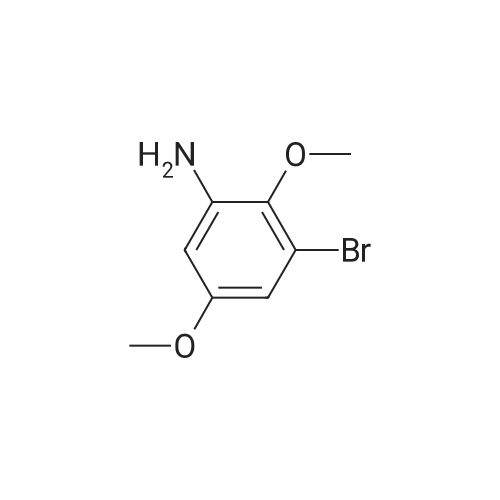
5-Bromo-2-methoxy-aniline A solution of 4-bromo-2-nitro-anisole (7.7 g, 33.1 mmol), triethylamine (4.6 ml, 33.1 mmol) and Raney Nickel catalyst (4 g) was vigorously stirred in ethanol (300 ml) under an atmosphere of hydrogen for 1 h at 20 C. After this time the theoretical amount of hydrogen had been absorbed (2.5 1), so the catalyst was filtered off and the solvent evaporated to afford the title compound as a light yellow solid (7 g, 104% yield), MS: m/e=201 (M+). intermediates for the preparation of benzylic amines |
|
With tin(ll) chloride; In ethanol; at 20℃; |
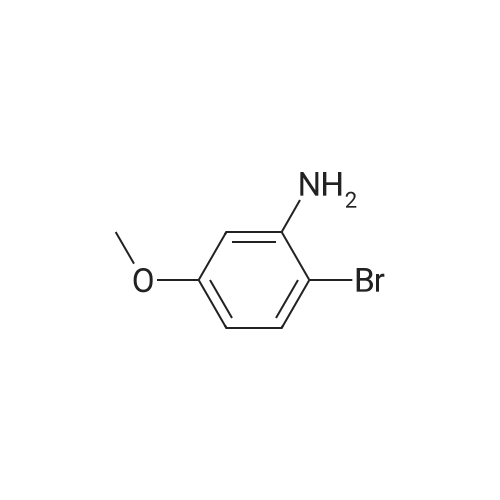
Example 159 2,6-Difluoro-N-(2-(methyloxy)-5-{3-[2-(1,2,3,4-tetrahydro-7-isoquinolinylamino)-4-pyrimidinyl]pyrazolo[1,5-a]pyridin-2-yl}phenyl)benzamide Step A: N-[5-Bromo-2-(methyloxy)phenyl]-2,2,2-trifluoroacetamide; To a solution of 4-bromo-2-nitroanisole (2.0 g, 0.009 mol) in absolute ethanol (100 mL) was added SnCl2.2H2O (11.68 g, 0.051 mol) and the resulting mixture was allowed to stir overnight at ambient temperature. The solvent was removed under reduced pressure, the residue was suspended in EtOAc (100 mL), washed with 1M NaOH (100 mL) and filtered through a celite pad. The organic layer was removed, concentrated by rotary evaporation, and dried under high vacuum. The resulting residue was then dissolved in DCM (150 mL) followed by the addition of triethylamine (5.19 g, 0.051 mol) and trifluoroacetic anhydride (4.52 g, 22 mmol). After overnight stirring, the reaction was washed with 1M HCl (50 mL), organic layer concentrated and purified by column chromatography (1-10% gradient of EtOAc in hexanes) to yield the title compound (1.53 g, 60%) as a white solid. ESIMS (M-H)-=297. |
|
With tin(ll) chloride; In ethanol; at 20℃; |
Step A: N-[5-Bromo-2-(methyloxy)phenyl]-2,2,2-trifluoroacetamide To a solution of 4-bromo-2-nitroanisole (2.0 g, 0.009 mol) in absolute EtOH (100 mL) was added SnCl2.2H2O (11.68 g, 0.051 mol) and the resulting mixture was allowed to stir overnight at rt. The solvent was removed under reduced pressure, residue suspended in EtOAc (100 mL), washed with 1M NaOH (100 mL), and filtered through a celite pad. The organic layer was removed, concentrated by rotary evaporation, and dried under high vacuum. The resulting residue was then dissolved in DCM (150 mL) followed by the addition of TEA (5.19 g, 0.051 mol) and TFAA (4.52 g, 0.022 mol). After overnight stirring, the reaction was washed with 1M HCl (50 mL), organic layer concentrated and purified by column chromatography (1-10% gradient of EtOAc in hexanes) to yield the title compound (1.53 g, 60%) as a white solid. ES-LC/MS m/z=297 [M-H]+. |
|
With hydrogenchloride; tin; In ethanol; water; at 20℃; for 5h; |
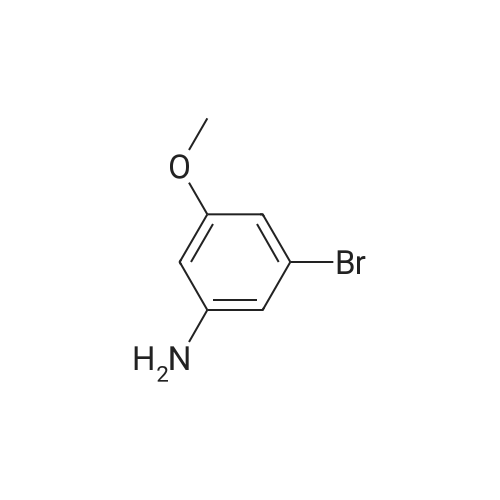
Step 2; Reduction of Nitro Group: Synthesis of 5-bromo-2-methoxyaniline 28.3 To a stirred solution of 28.2 (0.95 g, 4.09 mmol) in ethanol (30 mL) was added concentrated HCl (15 mL) and tin powder (0.95 g, 8 mmol). The reaction was stirred for 5 h. The solvent was then removed in vacuo and the acid was neutralised by the slow addition of 2.5M NaOH solution (13 mL) at 0 C. The aqueous mixture was then extracted with diethyl ether (3*50 mL). The organic fractions were dried over MgSO4, filtered and concentrated in vacuo to yield 28.3 as a brown solid (0.86 g, 100%). The product was not purified further. 1H NMR (CDCl3, 400 MHz) deltaH ppm: 3.85 (3H, s, OMe), 6.65 (1H, dd, J1=2 Hz, J2=10.12 Hz, ArH), 6.83 (1H, dd, J1=2.44 Hz, J2=8.2 Hz, ArH), 6.85 (1H, s, ArH) 13C NMR (CDCl3, 400 MHz) deltac ppm: 55.18 (ArC), 111.14 (ArCH), 112.75 (ArC), 116.87 (ArCH), 117.72 (ArC), 120.23 (ArCH), 137.15 (ArC), 145.90 (ArC); vmax (DCM)/cm-1: 3460.96, 3370.98, 1611.91, 1573.81; HRMS: calculated 201.9862, found 201.9855, molecular formula (C7H9BrNO).; Melting Point: 110 C. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping