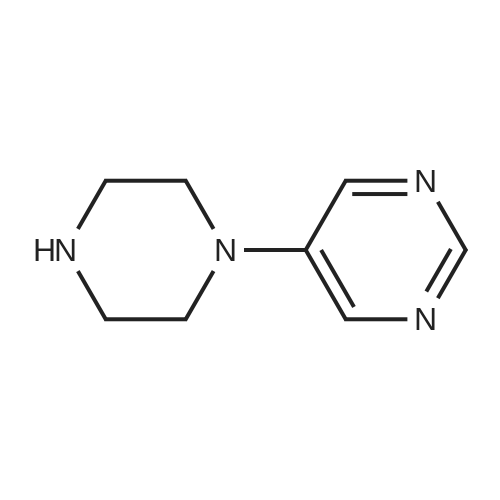
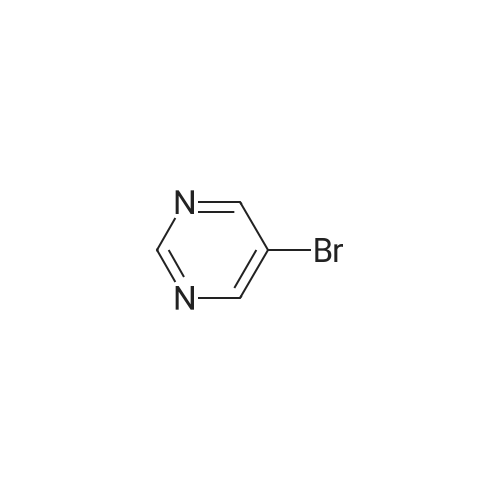
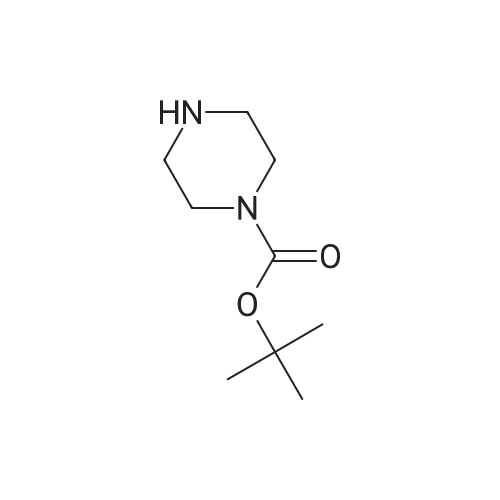
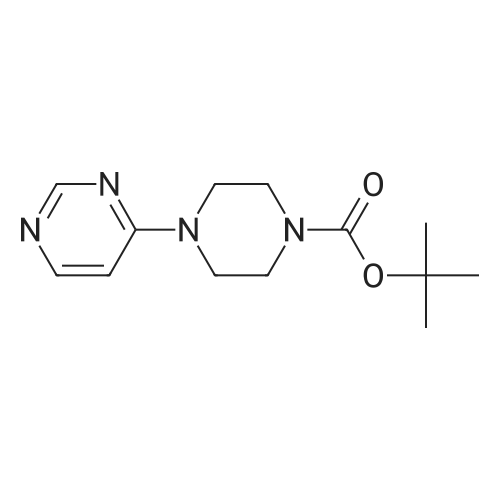
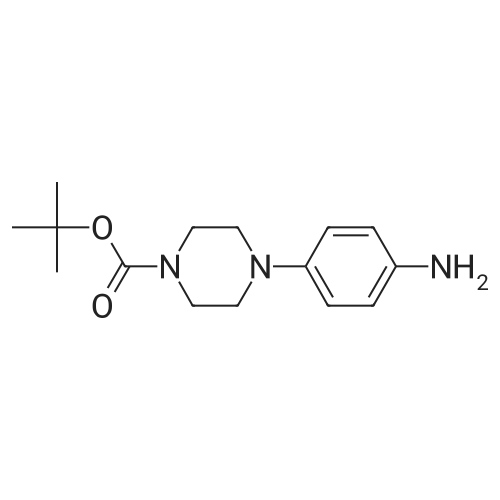
| 58.7% |
With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; In toluene; at 90℃; for 12h; |
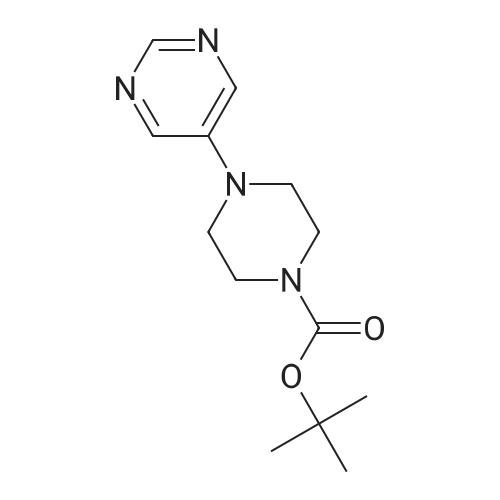
(1) Preparation of tert-butyl 4-(pyrimidin-5-yl)piperazin-1-carboxylate To a 100 mL eggplant-shaped bottle were added 5-bromopyrimidine (3.16 g, 20 mmol), tert-butyl piperazin-1-carboxylate (3.72 g, 20 mmol), 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl (2.49 g, 4 mmol), cesium carbonate (13.0 g, 40 mmol) and tris(dibenzylideneacetone)dipalladium (1.83 g, 2 mmol); and toluene (80 mL) was added. The reaction was carried out at 90Cunder the protection of nitrogen for 12 h. The mixture was filtrated under suction, the filtrate was concentrated, and the crude product was purified by silica gel column chromatography (dichloromethane: methanol=50:1) to get the title compound (3.1 g, yield: 58.7%). |
|
|
Argon is bubbled for 15 minutes into a mixture of 9.3 g of tert-butyl 1-piperazinecarboxylate, 7.95 g of 5-bromopyrimidine and 6.5 g of sodium tert-butoxide in 250 ml of toluene, which is then heated at reflux, 0.277 g of palladium acetate and 1.7 ml of tri-tert-butylphosphine are added and reflux is continued for 24 hours. 0.277 g of palladium acetate is added and the mixture is heated at reflux for 8 hours. The reaction mixture is cooled to AT, water is added, the mixture is subjected to extraction with AcOEt, the organic phase is filtered and dried over Na2SO4 and the solvent is evaporated under vacuum. The residue is chromatographed on silica gel, eluting with DCM, then with a DCM/AcOEt (50/50; v/v) mixture and finally with a DCM/MeOH (95/5; v/v) mixture. This gives 3.95 g of the expected product following recrystallization from a DCM/hexane/iso ether mixture. |
|
With tris-(dibenzylideneacetone)dipalladium(0); potassium tert-butylate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; In toluene; for 16h;Reflux; Inert atmosphere; |
General procedure: 2-Chloro-4-iodotoluene (250muL, 1.78mmol), 1-Boc-piperazine (398mg, 2.14mmol), Pd2(dba)3 (40.8mg, 0.045mmol), Xantphos (103mg, 0.178mmol) and potassium tert-butoxide (280mg, 2.50mmol) were dissolved in toluene (5mL) and heated at reflux for 16h under N2. The reaction was then concentrated and dissolved in EtOAc (20mL), filtered through celite and washed with additional EtOAc (50mL). The organic layer was washed with water (2×20mL) and brine (2×20mL), then dried with Na2SO4 and concentrated in vacuo. The crude residue was then purified by column chromatography (100% CyHex to 10% EtOAc/CyHex) to obtain the protected intermediate as an oil (436mg, 79%). MS, m/z=311 (100) [M+H]+, 313 (30). The intermediate was then dissolved in a 1:3 mixture of TFA/DCM (4mL) and stirred at 20C for 1h. The solvent was then evaporated in vacuo and the crude residue dissolved in EtOAc (10mL) which was then successively washed with a 10% NaHCO3 solution (10mL), water (10mL) and brine (10mL). The organic layer was then dried with Na2SO4 and concentrated in vacuo to obtain 106 as a solid (288mg, 97%). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping