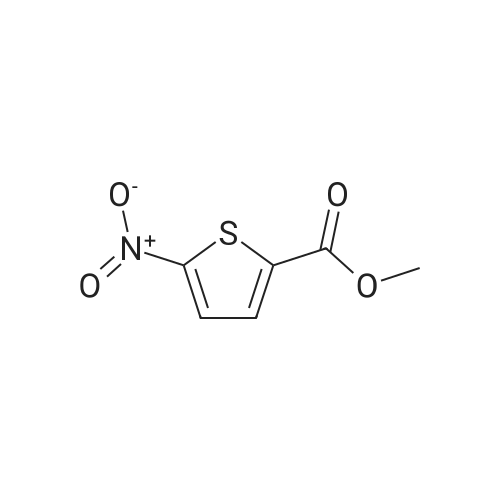
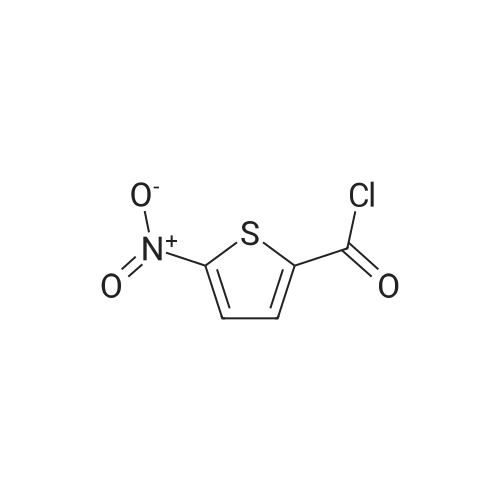
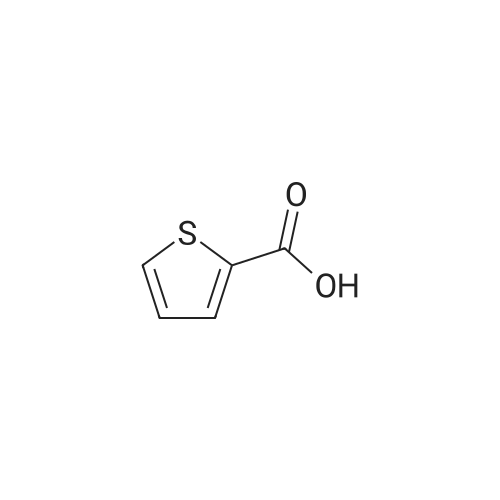
|
With oxalyl dichloride;N,N-dimethyl-formamide; In dichloromethane; at 20℃; for 1h; |
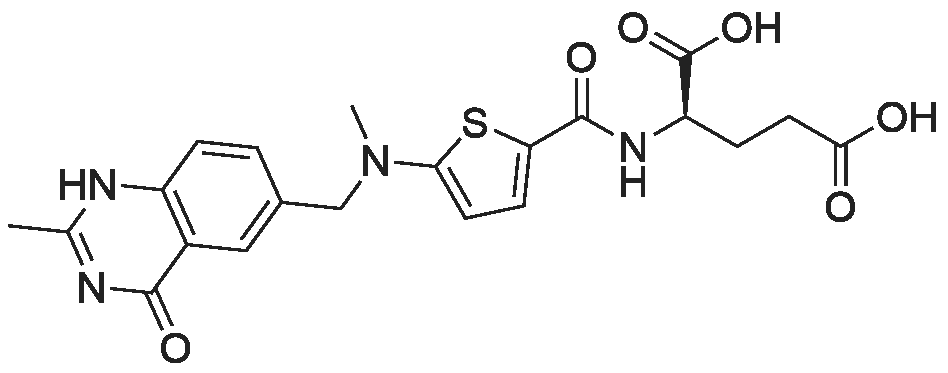
Example 247; Preparation of /V-f(3S)-1-r(3-hvdroxylphenyl)methvn-1-methyl-3- piperidiniumyl)-Lambda<f-r((5-r(cvclohexyloxy)carbonvpi-2-thienyl)amino)carbonvn- L-tyrosinamide trifluoroacetate; 5-Nitro-2-thiophenecarboxylic acid (2.8 g, 16 mmol) was suspended in methylene chloride (50 ml_). Oxalyl chloride in methylene chloride (2.0 M, 32.3 ml_) was added at room temperature followed by one drop of dimethyl formamide (0.1 mL). The reaction mixture was stirred at RT for 1 hr and concentrated. Methylene chloride (100 mL) was added, concentrated again and redissolved in methylene chloride (60 mL). N, N'-Dimethylaminopyridine (652 mg, 5.33 mmol) , triethyl amine(4.47 mL, 32 mmol) and cyclohexanol (2.54 mL, 24 mmol) were added to reaction mixture and stirred at room temperature overnight. The reaction mixture was filtered through a pad of silica gel (250 g), eluting with methylene chloride. Cyclohexyl 5-nitro-2-thiophenecarboxylate was obtained after concentration. LCMS (ESI) 256 [M+H]+. |
|
With thionyl chloride; for 0.5h;Cooling with ice; Reflux; Inert atmosphere; |
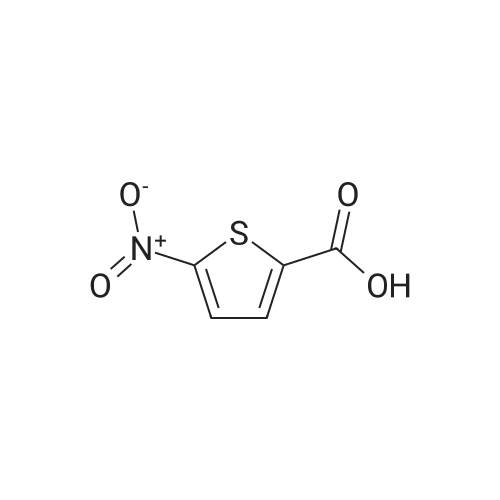
General procedure: General method: According to the method described in literature,8,9 <strong>[6317-37-9]5-nitrothiophene-2-carboxylic acid</strong> (1.0?g, 5.8?mmol) was carefully added to 6?ml of SOCl2 in a ice-cooled round bottom flask. The solution was refluxed and stirred under N2 for 30?min, cooled and evaporated under reduced pressure to obtain the corresponding acyl chloride as a sticky solid. The solid was dissolved into dry CH2Cl2 (10?ml), cooled in ice-bath and the suitable amine (5.8?mmol) was slowly added followed by TEA (0.89?ml, 6.44?mmol). The resulting mixture was stirred under N2 atmosphere at rt for 15?min, then CH2Cl2 was evaporated under reduced pressure and the residue partitioned between AcOEt and 2?N NaOH. The organic layer was washed in succession with water, 0.5?N HCl, brine, and then dried over anhydrous Na2SO4. The solvent was evaporated to dryness and the resulted solid residue was washed with the indicated solvent and used directly in the next step. |
|
|
5-Nitrothiophene-2-carboxylic acid (2.0g) was taken in a 100mL single neck RB flask equipped with N2-inlet, to this was added CH2Cl2 (30mL), OxallylChloride (6.0mL, 3V) at 0°C and stirred for few minutes. Then DMF (few drops) was added slowly (drop wise) at same temperature until evolution of bubbles in the reaction mixture was stopped, and allowed to stir at room temperature for 3h. The reaction mixture was concentrated re-dissolved in dry CH2Cl2 and used in next step. |
|
With thionyl chloride; In benzene; at 0℃; for 0.5h; |
General procedure: To the solution of heterocyclic acid (1 mmol) in benzene at 0°C was added SOCl2 (1.5 mmol) and reaction mixture was stirred for 30 min. Solvent was evaporated and heterocyclic acid chlorides obtained were used for next step without any purification. |
|
With thionyl chloride; for 3h;Reflux; |
10 g of <strong>[6317-37-9]5-nitro-2-thiophenecarboxylic acid</strong> and 20 ml of thionyl chloride were added to the reaction flask. The mixture was refluxed for 3 hours, and the reaction mixture was transferred to a one-neck flask, concentrated to a liquid-free flow, and dissolved in 30 ml of dichloromethane. 13.8 g of diethyl L-glutamate, 20.6 g of triethylamine, 50 ml of dichloromethane were added to a 150 ml three-necked flask, and the mixture was stirred and cooled to 0 to 5 ° C. The concentrated dichloromethane solution of the concentrate was slowly added dropwise. After the completion of the dropwise addition, the reaction was kept for 1 hour. After completion of the reaction, it was washed successively with 100 ml of 2 mol/L hydrochloric acid and 100 ml of a saturated sodium hydrogencarbonate solution. Separating the organic phase, The residue was dried over anhydrous sodium sulfate, and then concentrated to yield 16.53 g of ethyl 2-(5-nitro-2-thiopheneyl)-L-glutamic acid, yield 79.9percent. |
|
With oxalyl dichloride; In tetrahydrofuran; dichloromethane; at 20℃; for 4h; |
REFERENCE EXAMPLE 15; 2-Amino-5-methySarninocarbonylthiophene; a) 5-Nitrothiophepie-2-carbonyl chloride; 2.89 mL (5.78 mmol) of 2.0 M oxaiyl chloride solution in CH2CI2 were added to a solution of 2-nitro-5-thiophene carboxylic acid (500 mg, 2.89 mmol) in THF (29 mL). The reaction mixture was stirred at room temperature for 4 h. The solvent was then evaporated to dryness, and the desired compound was obtained and used in next step without further purification. |
|
With thionyl chloride; In methanol; at 75 - 80℃; for 2h; |
Put 30g of <strong>[6317-37-9]5-nitro-2-thiophenecarboxylic acid</strong> into 50ml of thionyl chloride100ml three-necked bottle,Warming up,After controlling the internal temperature at 75 ° C ~ 80 ° C for 2 h,Take about 1 ml of the reaction solution.Derivatized by adding 2 ml of anhydrous methanol,Taking <strong>[6317-37-9]5-nitro-2-thiophenecarboxylic acid</strong> as a control,TLC detection (ethyl acetate: n-hexane = 1:1) to the end of the reaction.Transfer the reaction solution to a 250 ml single-mouth bottle.Concentrated to dryness,Add 30 ml of toluene to dryness under reduced pressure.Get green needle crystals. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping