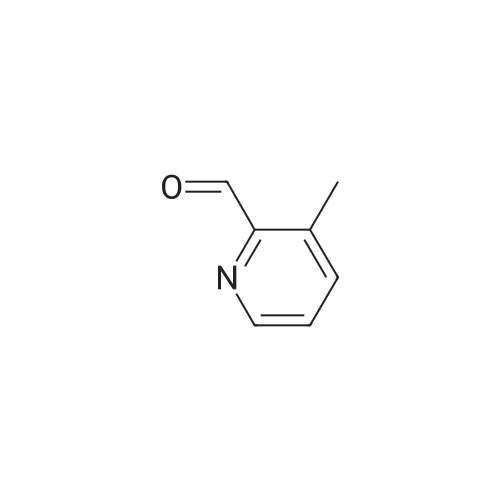
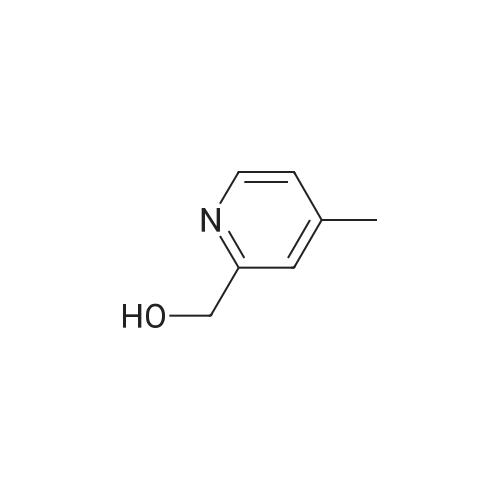
| 80% |
With manganese(IV) oxide; In 1,2-dichloro-ethane; at 50℃; for 6h; |
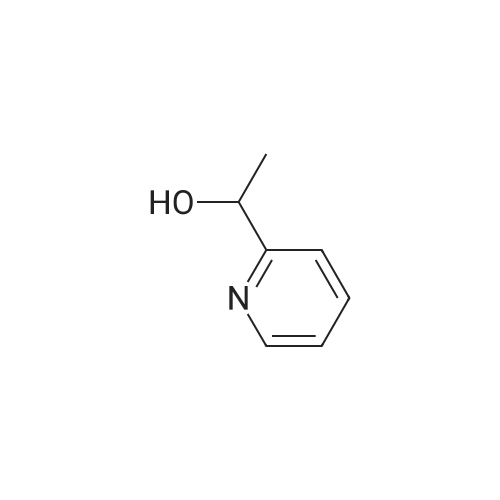
General procedure: Hydroxy-methylpyridine 3b, 7, or 13, 1.9 mmol, was dissolved in 25 mL of 1,2-dichloroethane, 1.65 g (19 mmol) of activated manganese dioxide was added, and the mix-ture was stirred for 6 h at 50°C. The precipitate was filtered off, the filtrate was evaporated under reduced pressure, and the residue was used in the next step without additional purification. 3-Methylpyridine-2-carbaldehyde (1c). Yield 185 mg (1.52 mmol, 80percent), light oily material. 1 H NMR spectrum (CDCl 3 ), delta, ppm: 2.66 s (3H, Me), 7.39 d.d (1H, 5-H, J = 7.8, 4.6 Hz), 7.62 d (1H, 4-H, J = 7.8 Hz), 8.66 d (1H, 6-H, J = 4.6 Hz), 10.20 s (1H, CHO). Mass spectrum, m/z 122.06 (I rel 100percent) [M + H] + . Calculated: M 122.06. |
|
With manganese(IV) oxide; In chloroform; at 70℃; for 2h; |
A portion (605.3 mg) of the obtained product was dissolved in chloroform (30 ml) and then added with manganese dioxide (3.03 g) (chemicals treated, manufactured by Wako Pure Chemical Industries, Ltd.), followed by stirring at 70°C for 2 hours. After completion of reaction, the catalyst was removed by filtration with Celite and the solvent was concetrated. The residue was purified by silica gel column chromatography (chloroform/ethyl acetate=1:1), to thereby obtain a light orange liquid of the subject compound (419.8 mg). MS (FAB,Pos.) :m/z= 122 [M+1]+1H-NMR(500MHz,CDCl3) : delta=2.67(3H,s),7.40(1H,dd,J=7.8,4.6Hz),7.64(1H,d,J=7.8Hz) ,8.67(1H,d,J=4.6Hz) ,10.2(1H,s) . |
|
|
Commercially available 2,3-lutidine (5.00 g) was dissolved in dichloromethane (50 ml) and the solution was cooled to 0°C. After that, the solution was added with meta-chloroperbenzoic acid (12.1 g) and then stirred at room temperature for 2 hours. After completion of the reaction, the solution was added with dichloromethane and washed with a 1 mol/l sodium hydroxide aqueous solution and saturated saline solution, followed by drying with anhydrous sodium sulfate. Then, the solvent was distilled off, thereby obtaining crude 2,3-lutidin-N-oxide (3.16 g). A 2.00 g part thereof was dissolved in dichloromethane (40 ml) and then the solution was cooled to 0°C. Subsequently, the solution was added with trifluoroacetic anhydride (4.49 ml), followed by stirring at room temperature for 4 hours and then at 45°C for 3 hours. After completion of the reaction, the solvent was distilled off and the residue was dissolved in methanol (30 ml), followed by the addition of a sodium methoxide/methanol solution until the pH of the solution would reach pH = 10. After the solution had been stirred at room temperature for 1 hour, the solvent was distilled off and extraction was then carried out with dichloromethane. The extract was dried with anhydrous sodium sulfate and the solvent was then distilled off, thereby obtaining 3-methyl-2-hydroxymethylpyridine (1.30 g). A 605.3 mg part thereof was dissolved in chloroform (30 ml) and then added with manganese dioxide (chemically processed product) (3.03 g), followed by stirring at 70°C for 2 hours. After completion of the reaction, the catalyst was removed through Celite filtration and the solvent was then concentrated. Then, the residue was purified through silica gel column chromatography (chloroform/ethyl acetate), thereby obtaining the subject compound (419.8 mg) as a pale-orange colored liquid. MS(FAB,Pos.):m/z=122[M+H]+1H-NMR(500MHz,CDCl3):delta=2.67(3H,s),7.40(1H,dd,J=7.8,4.6Hz),7.64(1 H,d,J=7.8Hz),8.67(1H,d,J=4.6Hz),10.2(1H,s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping