Alternatived Products of [ 629-82-3 ]
Product Details of [ 629-82-3 ]
| CAS No. : | 629-82-3 |
MDL No. : | MFCD00009563 |
| Formula : |
C16H34O
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | NKJOXAZJBOMXID-UHFFFAOYSA-N |
| M.W : |
242.44
|
Pubchem ID : | 12399 |
| Synonyms : |
|
Application In Synthesis of [ 629-82-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 629-82-3 ]
- 1
-
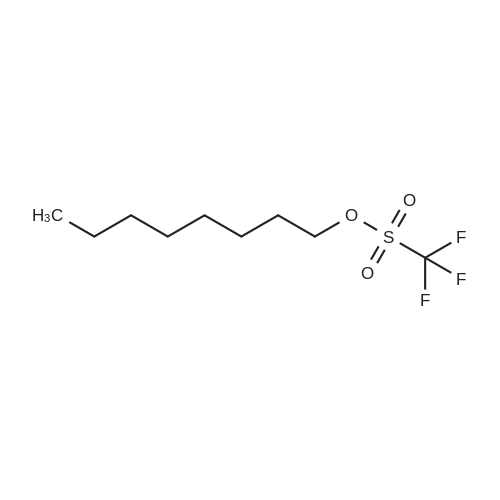 [ 71091-89-9 ]
[ 71091-89-9 ]

-
dicyclohexylammonium α-cyanoacrylate
[ No CAS ]

-
n-octyl 2-cyanoacrylate
[ No CAS ]

-
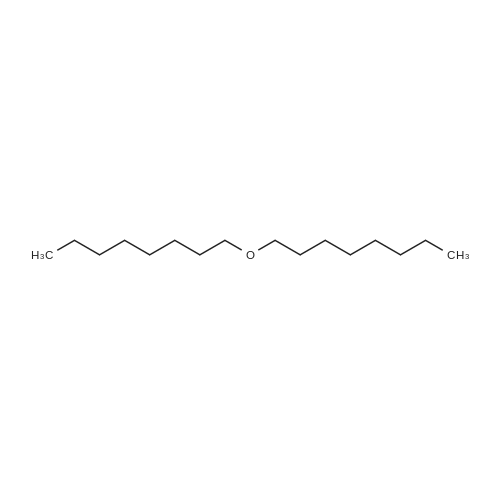 [ 629-82-3 ]
[ 629-82-3 ]

-
 [ 1215021-90-1 ]
[ 1215021-90-1 ]

-
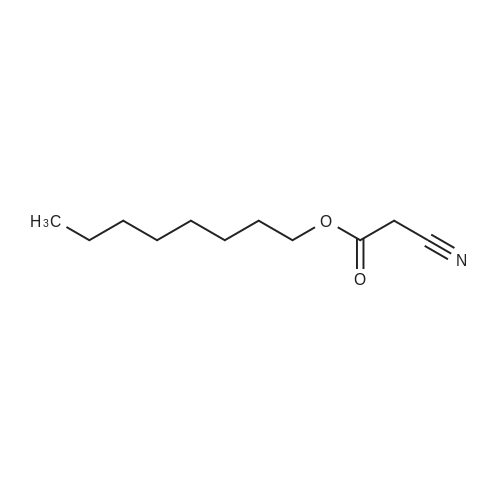 [ 15666-97-4 ]
[ 15666-97-4 ]

-
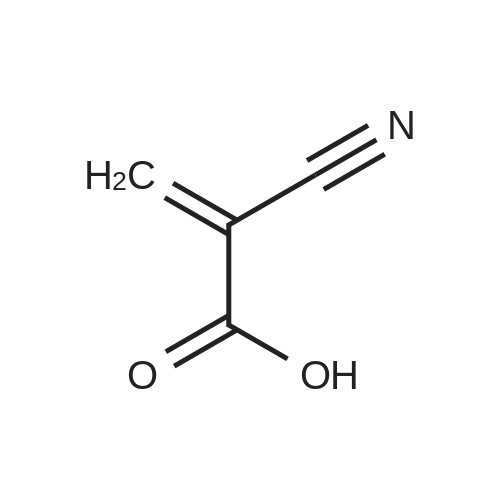 [ 15802-18-3 ]
[ 15802-18-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 65% |
With trifluoroacetic acid; In dichloromethane; at 22℃;Product distribution / selectivity; |
To a solution of octyl triflate (0.05 mol) in dry dichloromethane (DCM, 100 mL) was added trifluoroacetic acid [TFA] (0.004 mol) and t-butylated hydroxyanisole (0.25 wt%). A solution of Dicyclohexylammonium alpha-cyanoacrylate in dry DCM (80mL) was added dropwise (2h). The resulting solution was stirred at 22 C overnight. NMR showed approx 10% of the triflate remaining with no trace of polymer. A solution of 0.078 mol of Dicyclohexylammonium alpha-cyanoacrylate in dry DCM (20 mL) was added. The mixture was stirred at 22 C until triflate had disappeared in the NMR (4h). The mixture was acidified (TFA) and reduced in vacuo. The precipitated solid was removed by filtration. Hexane (100 mL) was added. The mixture was reduced and filtered again. This was repeated. The solvents were removed to afford 1 1.5g of a yellow liquid which was -75- 80% CA monomer. The impurities are cyanoacrylic acid (due to the excess ofDicyclohexylammonium alpha-cyanoacrylate used), dioctyl ether (a byproduct carried through from the triflate formation) a small amount (<2%) of polymer, trace amounts of the amine triflate salt, octyl cyanoacetate (formed from cyanoacetic acid amine salt, a residue from incomplete formation of the Dicyclohexylammonium alpha-cyanoacrylate). There are no byproducts or side reaction evident from the CA formation reaction. The octyl CA was distilled to purify and afforded an overall yield of 65% based on triflate. The synthesis procedure is general and has been used to prepare crude samples of other monomers such as n-propyl CA, 3-methoxybutyl CA and bis-cyanoacrylic acid ester of PEG 400.[0060] NMR Analysis of distilled material: 1H NMR (CDCI3) delta : 7.05, (s, 1 H, =CHH); 6.65 (s, 1 H, =CHH); 4.27 (t, 2H, ~COOCH2CH2~); 1.73 (m, 2H, ~COOCH2CH2~); 1 .40-1.25 (br m, 10H, ~COOCH2CH2CH2CH2 CH2CH2CH2~); 0.88 (t, 3H, ~CH2CH3).[0061] 13C 5 : 163.27 ~COO~; 143.3, =CH2; 1 14.5, ~CN; 1 13.4, ~C=CH2; 66.99, ~COOCH2~; 31 .8; 29.2; 28.4; 25.8; 24.7; 22.7 (~CH2CH3); 14.1 1 (~CH3). |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









