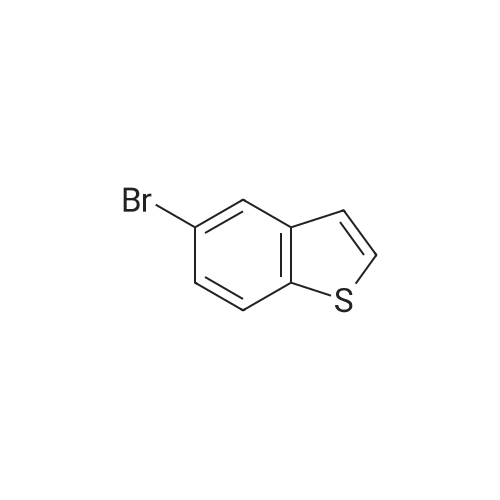
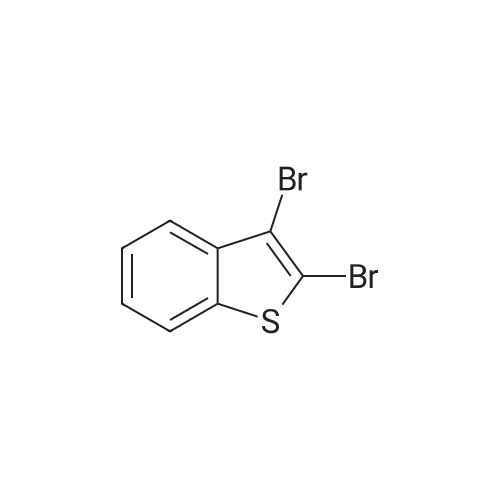
| 84% |
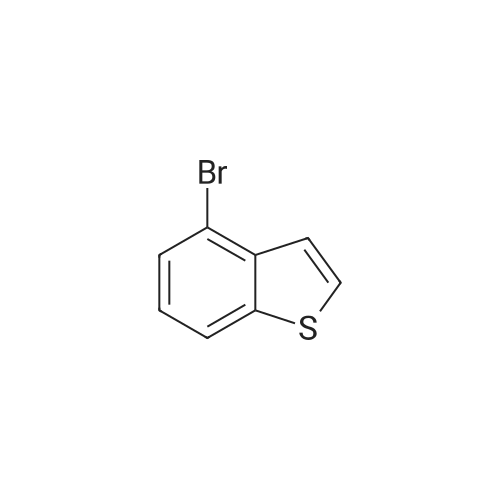
With bromine; potassium acetate; In dichloromethane; at 20℃;Reflux; |
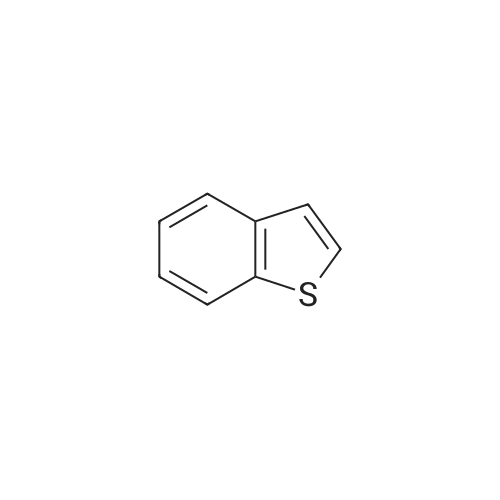
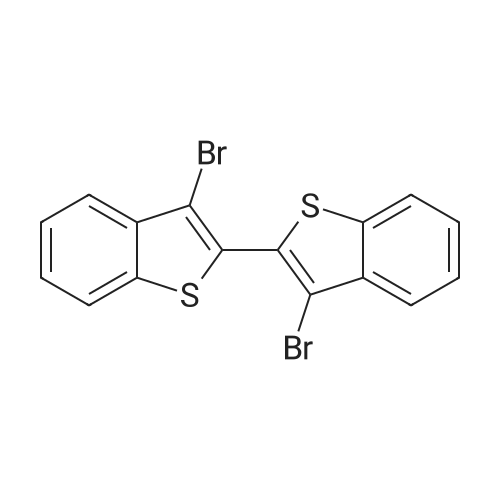
To a CH2Cl2 solution (50 mL) of benzo[b]thiophene (14, 5.00 g, 37.3 mmol) and KOAc (7.30 g, 74.6 mmol) was added Br2 (3.8 mL, 74.6 mmol) at 20 C, and the solution was heated under reflux for 24 h. To the solution was added a satd solution of Na2S2O3 and NaHCO3. The organic and the aqueous layer were separated, and the latter was extracted with CH2Cl2 (3*30 mL). The combined organic layers were dried (Na2SO4), filtered, and concentrated in vacuo. The residue was purified by flash silica column chromatography (pure heptanes) to yield 15a as a white solid (9.1 g, 84%). The spectroscopic data were identical with those reported.<ce-sup primary_key="ce-sup-35854493-none">17,18 |
| 80% |
With bromine; In chloroform; at 20℃; for 24h; |
Chloroform (200 mL) was used to dissolve benzo[b]thiophene(140 mmol) followed by drop wise addition of Br2 (310 mmol). The mixture was stirred at room temperaturefor 24 h. Washing of the resultant solution was done withNa2S2O3 aqueous solution and organic layer was separatedby using ethyl acetate. It was dried and concentrated withanhydrous MgSO4. The product was re-precipitated usingn-hexane in excess which resulted in the formation of whitecolored product. |
| 16.5 g (57 mmol, 28%) |
With bromine; In chloroform; |
A. 2,3-Dibromobenzo[b]thiophene STR16 Benzothiophene (26.8 g, 0.2 mol) was dissolved in 150 mL CHCl3 and treated with a solution of bromine (64 g, 0.4 mol) in 75 mL CHCl3 dropwise over an hour. The reaction was allowed to stir overnight then cautiously quenched with saturated aqueous Na2 CO3 until no gas evolution was evident. The layers were separated and the organic layer was first washed with saturated aqueous Na2 CO3 then with water. It was dried over MgSO4 and concentrated under vacuum to a solid. Recrystallized from MeOH to obtain 16.5 g (57 mmol, 28%) of a white fluffy solid. 1 H NMR (CDCl3) δ7.77-7.71 (m, 2H), 7.46-7.38 (m, 2H). |
|
With bromine; In chloroform; |
A. Preparation of 2,3-dibromobenzo[b]thiophene. A solution of 64 g of bromine in 50 ml of chloroform was added to a solution of 26.8 g of 1-benzothiophene in 150 ml of chloroform. After stirring for approximately 18 hours, the reaction mixture was washed sequentially with 0.1N sodium hydroxide, 0.1N aqueous sodium thiosulfate, and water. The organic layer was dried over magnesium sulfate and evaporated to dryness. The residue was crystallized twice from methanol to provide 20.54 g of the desired subtitled intermediate, m.p. 57-59 C. Analysis for C8 H4 Br2 S; Calculated: C, 32.91; H, 1.38; Found: C, 32.72; H, 1.49. |
| 16.5 g (57 mmol, 28%) |
With bromine; In CHCl3and; CHCl3dropwise; |
A. 2,3-Dibromobenzo[b]thiophene. Benzothiophene (26.8 g, 0.2 mol) was dissolved in 150 mL CHCl3and treated with a solution of bromine (64 g, 0.4 mol) in 75 mL CHCl3dropwise over an hour. The reaction was allowed to stir overnight then cautiously quenched with saturated aqueous Na2CO3until no gas evolution was evident. The layers were separated and the organic layer was first washed with saturated aqueous Na2CO3then with water. It was dried over MgSO4and concentrated under vacuum to a solid. Recrystallized from MeOH to obtain 16.5 g (57 mmol, 28%) of a white fluffy solid. 1H NMR (CDCl3) δ 7.77-7.71 (m, 2H), 7.46-7.38 (m, 2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping