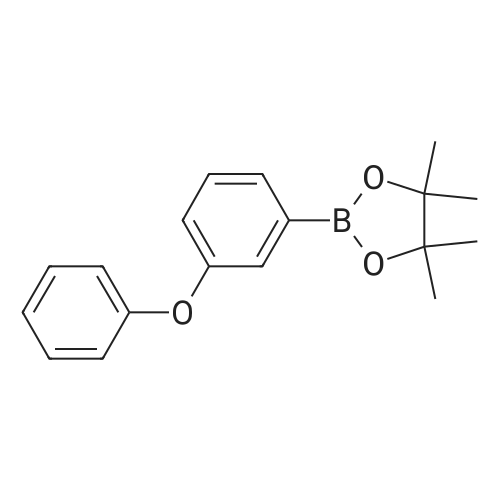
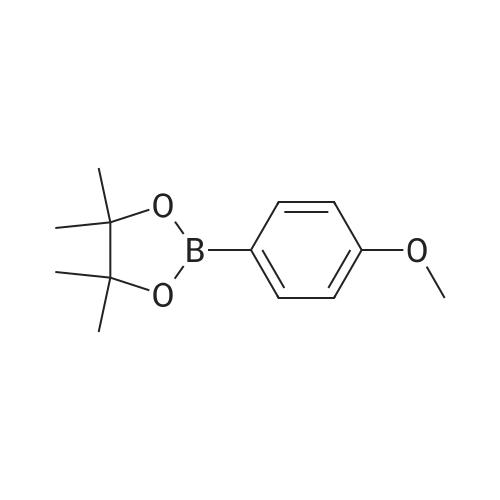
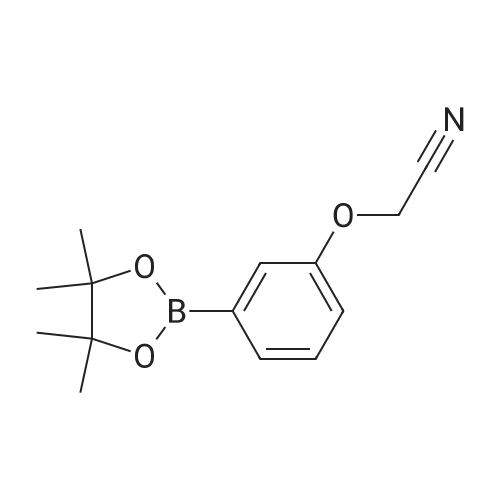
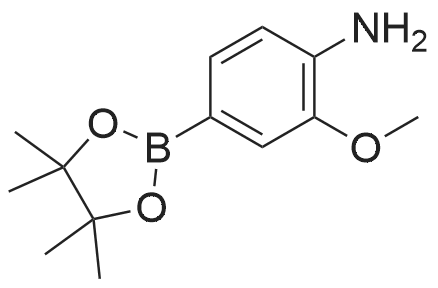
| 42.4% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate In 1,4-dioxane at 90℃; for 16 h; Inert atmosphere |
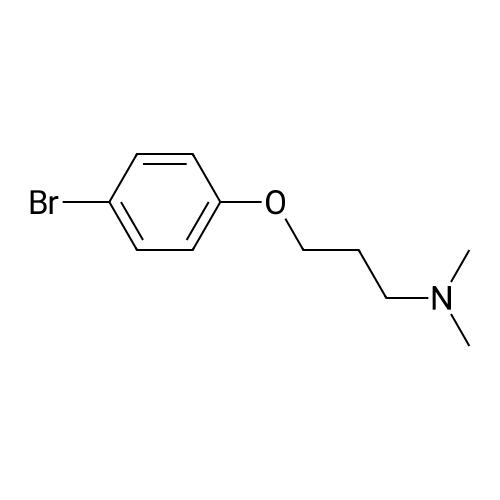
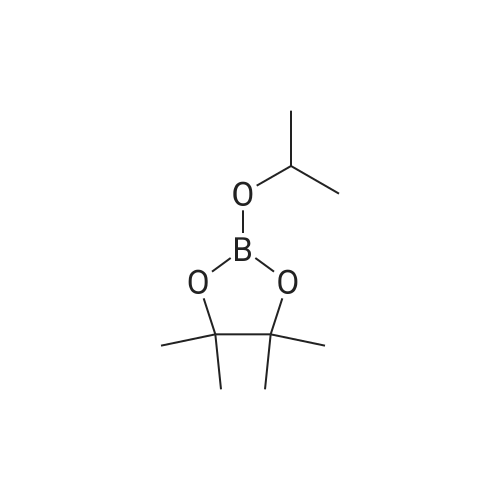
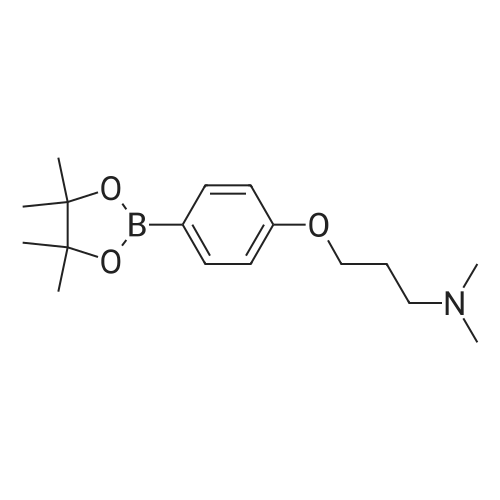
NrV-Dimethyl-3-[4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenoxy]propan-l- amine N,N-Dimethyl-3-[4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)phenoxy]propan-l -amine is available commercially from several suppliers including Apollo Scientific Ltd., Whitefield Rd, Bredbury, Stockport, Cheshire, SK6 2QR, UK. CAS number [627899-90- 5], catalogue number OR12268. Alternatively, it can be prepared as follows: A 1 : 1 complex of [l, -bis(diphenylphosphino)ferrocene]dichloropalladium(II) with dichloromethane (8.64 mg, 10.58 μιηο) was added to 3-(4-bromophenoxy)-N,N- dimethylpropan-1 -amine (546 mg, 2.12 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2- dioxaborolane) (644 mg, 2.54 mmol) and potassium acetate (830 mg, 8.46 mmol) in 1,4- dioxane (6 mL) warmed to 90°C under nitrogen. The resulting suspension was stirred at 90 °C for 16 h. The reaction mixture was evaporated to dryness and re-dissolved in DCM (25 mL), and washed with water (20 mL). The organic layer was dried with a phase separating cartridge, filtered and evaporated to afford crude product. The crude product was purified by FCC, elution gradient 0 to 10percent MeOH in DCM. Pure fractions were evaporated to dryness to afford the desired material as a brown waxy solid (274 mg, 42.4 percent). NMR Spectrum: NMR (500MHz, CDCls) δ 1.33 (12H, s), 1.89 - 2.08 (2H, m), 2.32 (6H, s), 2.53 (2H, dt), 4.05 (2H, t), 6.86 - 6.91 (2H, m), 7.71 - 7.76 (2H, m). Mass Spectrum: m/z (ES+)[M+H]+ = 258. |
| 42.3% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate In 1,4-dioxane at 90℃; for 16 h; Inert atmosphere |
Dichloro [1,1 ‘-bis(diphenylphosphino)ferrocene]palladium (II) dichloromethane adduct(0.063 g, 0.08 mmol) was added to 3-(4-bromophenoxy)-N,N-dimethylpropan-1-amine(2g, 7.75 mmol), 4,4,4’,4’,5 ,5 ,5’,5 ‘-octamethyl-2,2’-bi(1 ,3 ,2-dioxaborolane) (2.36 g, 9.30 mmol) and potassium acetate (3.04 g, 30.99 mmol) in 1,4-dioxane (35 mL) and the mixture degassed for 15 minutes. The resulting suspension was stirred at 90 °C for 16 hours under an inert atmosphere. The reaction mixture was evaporated to dryness, redissolved in DCM(25 mL), washed with water (20 mL) and the organic layer was dried with a phase separating cartridge, filtered and evaporated. The crude product was purified by flash silica chromatography, elution gradient 0 to 10percent MeOH in DCM, to afford the desired material as a brown oil (1.000 g, 42.3 percent) which solidified on standing. NMR Spectrum: ‘H NMR (400MHz, CDC13) ? 1.33 (12H, s), 1.96 - 2.07 (2H, m), 2.34(6H, s), 2.52 - 2.65 (2H, m), 4.04 (2H, t), 6.83 - 6.94 (2H, m), 7.68 - 7.78 (2H, m).Mass Spectrum: mlz (ES+) [M+H]+ = 306 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping