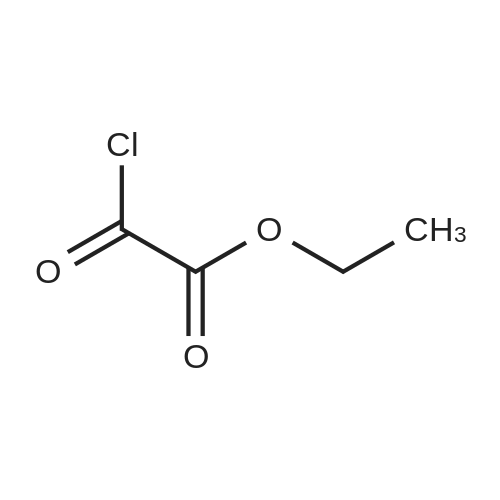
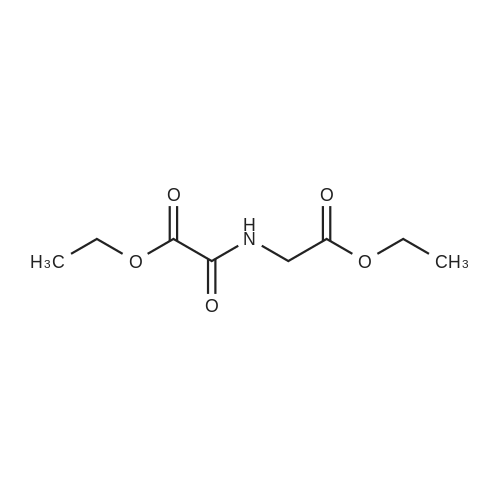
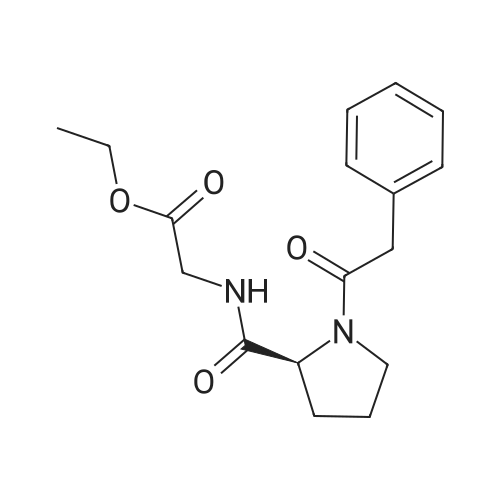
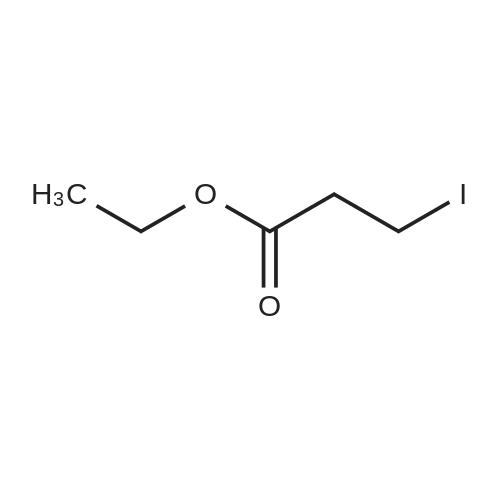
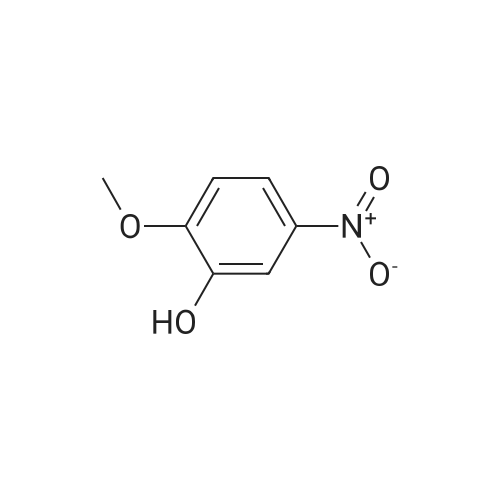
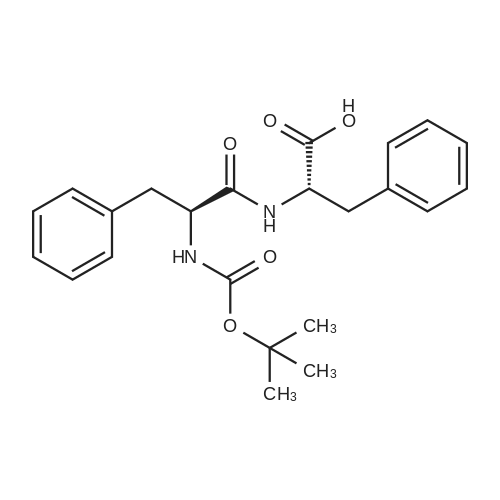
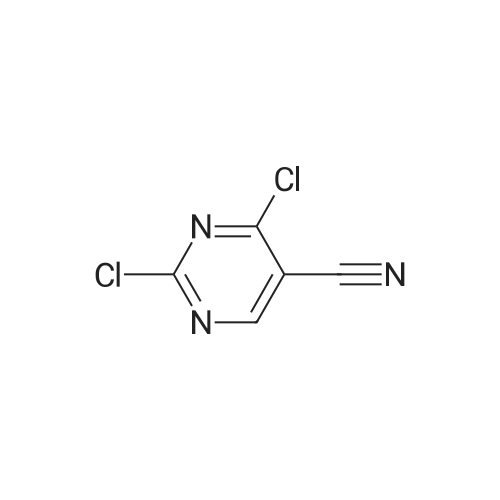
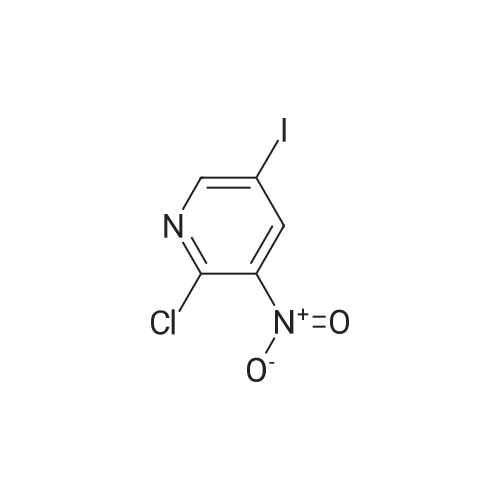
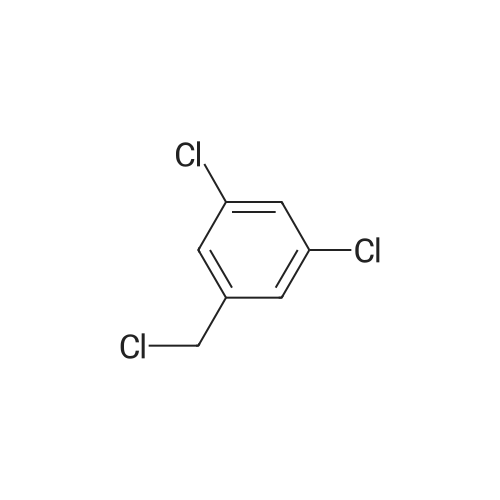
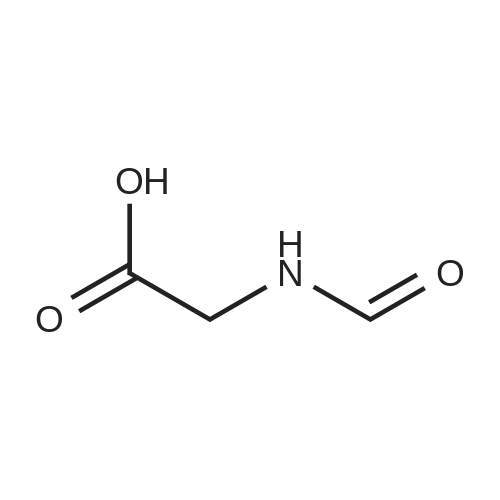
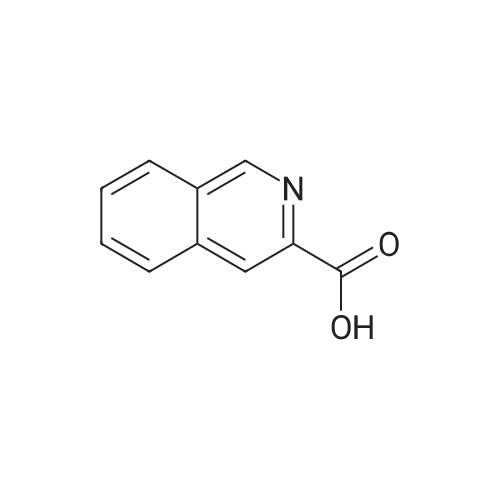
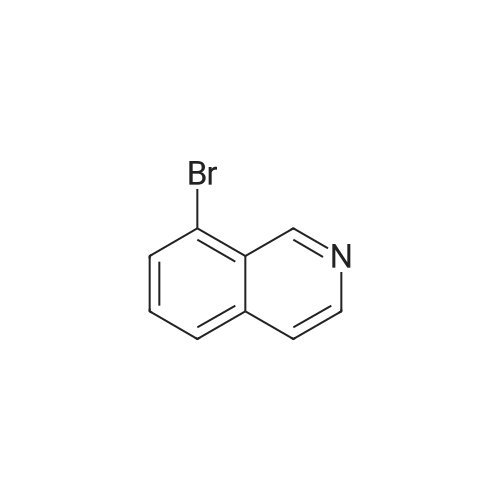
| 96% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine; In N,N-dimethyl-formamide; at 20℃;Molecular sieve; Inert atmosphere; |
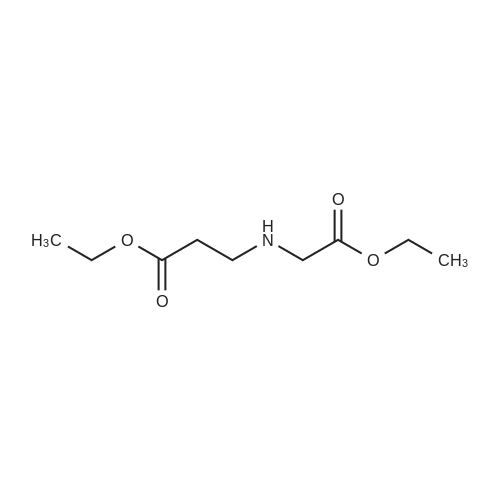
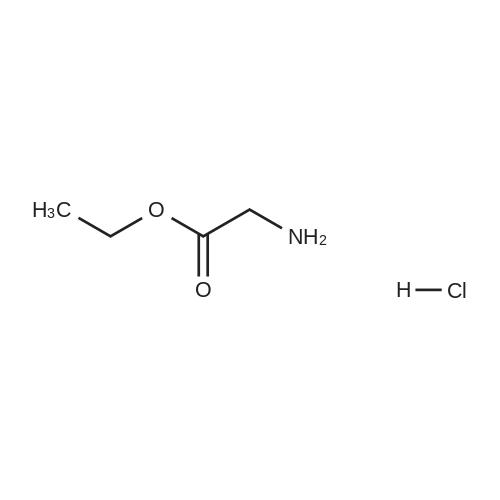
On an ice-water bath, a solution of 3-isoquinolinecarboxlic acid (236 mg, 1.36 mmol) in DMF (4 mL), was added EDCI (392 mg, 2.04mmol), HOBt (276 mg, 2.04 mmol), molecular sieves, and TEA (665 muL, 4.77 mmol) with N2protection. Fifteen minutes later, a solution of glycine ethyl ester hydrochloride (190 mg, 1.36mmol) in DMF (1.5 mL) was added dropwise. After stirring at ambient temperature overnight,the resulting mixture was filtered through celite. The filtrate was concentrated under reducedpressure to remove DMF. The residue was added brine (15 mL), taken up with CH2Cl2 (15 mL ×3). The combined organic layers were dried, and concentrated. The residue was purified withchromatography using EtOAc/petroleum ether (1/3) as eluent to give 339 mg white solid, in 96percentyield. 1H NMR (400 MHz, CDCl3) 9.18 (s, 1H), 8.69 (brs, 1H), 8.60 (s, 1H), 8.03 (d, J = 7.92Hz, 1H), 7.98 (d, J = 8.12 Hz, 1H), 7.76 (dt, J = 1.12 Hz, 7.52 Hz, 1H), 7.70 (dt, J = 1.06 Hz,7.47 Hz, 1H), 4.33 (d, J = 5.64 Hz, 2H), 4.27 (q, J = 7.13 Hz, 2H), 1.32 (t, J = 7.12 Hz, 3H); 13CNMR (100 MHz, CDCl3) delta 169.82, 165.11, 151.28, 143.13, 135.91, 131.02, 129.82, 128.91,128.13, 127.65, 120.48, 61.46, 41.54, 14.18. IR (diamond, cm-1) numax 3346.8, 1742.4, 1663.3, 1532.3, 1187.9, 910.2, 746.8. mp 146147 °C. |
| 96% |
|
On an ice-water bath, a solution of 3-isoquinolinecarboxlic acid (236 mg, 1.36mmol) in DMF (4 mL), was added EDCI (392 mg, 2.04 mmol), HOBt (276 mg, 2.04mmol), molecular sieves, and TEA (665 muL, 4.77 mmol) with N2protection. Fifteen minutes later, a solution of glycine ethyl esterhydrochloride (190 mg, 1.36 mmol) in DMF (1.5 mL) was added dropwise. Afterstirring at ambient temperature overnight, the resulting mixture was filteredthrough celite. The filtrate was concentrated under reduced pressure to removeDMF. The residue was added brine (15 mL), taken up with CH2Cl2(15 mL × 3). The combined organic layers were dried, and concentrated. Theresidue was purified with chromatography using EtOAc/petroleum ether (1/3) aseluent to give 339 mg white solid, in 96percent yield. 1H NMR (400 MHz, CDCl3) d 9.18 (s, 1H), 8.69 (brs,1H), 8.60 (s, 1H), 8.03 (d, J = 7.92Hz, 1H), 7.98 (d, J = 8.12 Hz, 1H),7.76 (dt, J = 1.12 Hz, 7.52 Hz, 1H),7.70 (dt, J = 1.06 Hz, 7.47 Hz, 1H),4.33 (d, J = 5.64 Hz, 2H), 4.27 (q, J = 7.13 Hz, 2H), 1.32 (t, J = 7.12 Hz, 3H); 13C NMR (100 MHz, CDCl3) delta169.82, 165.11, 151.28, 143.13, 135.91, 131.02, 129.82, 128.91, 128.13, 127.65, 120.48, 61.46, 41.54, 14.18. IR(diamond, cm-1) numax 3346.8, 1742.4, 1663.3,1532.3, 1187.9, 910.2, 746.8. mp 146-147 °C |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping