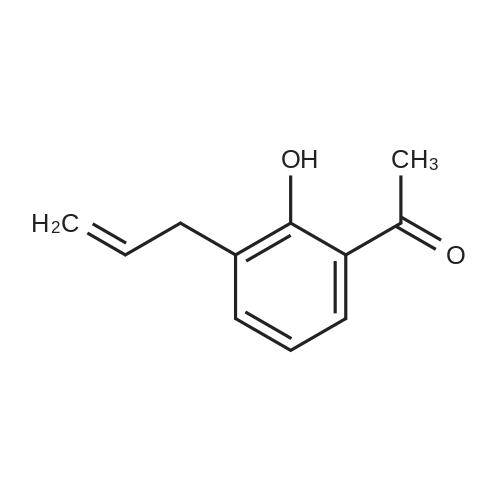
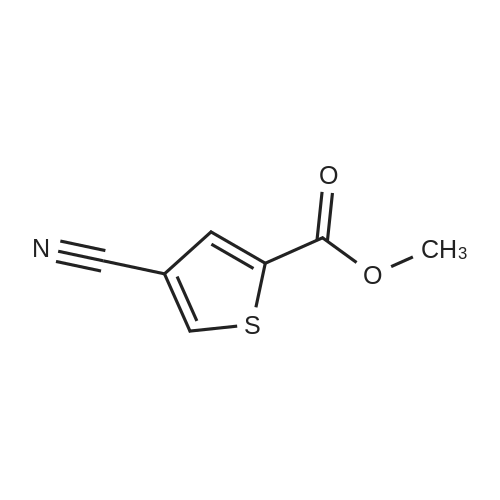
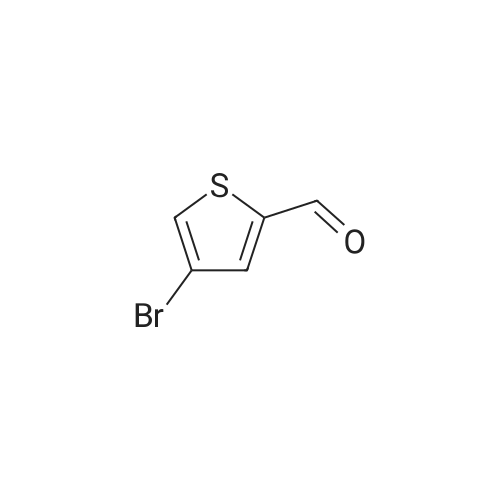
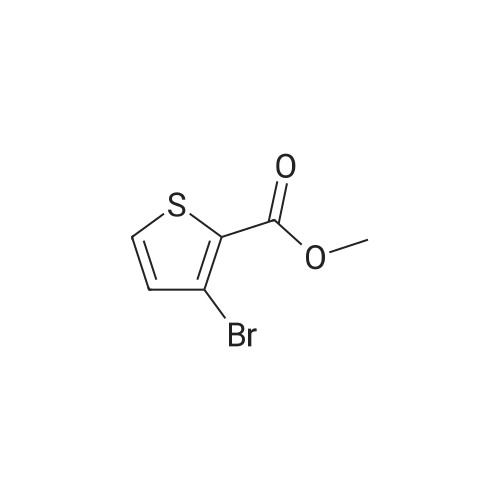
| 91.4% |
With thionyl chloride; for 6h;Reflux; |
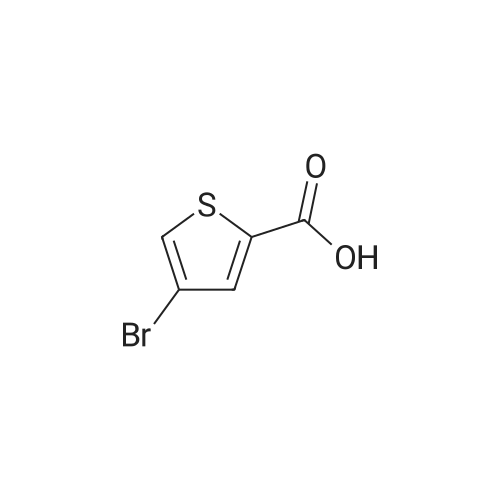
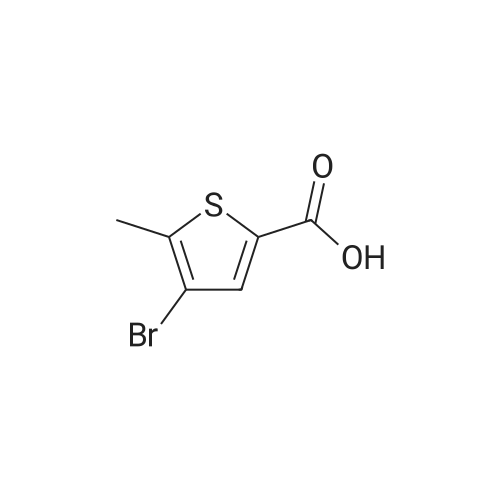
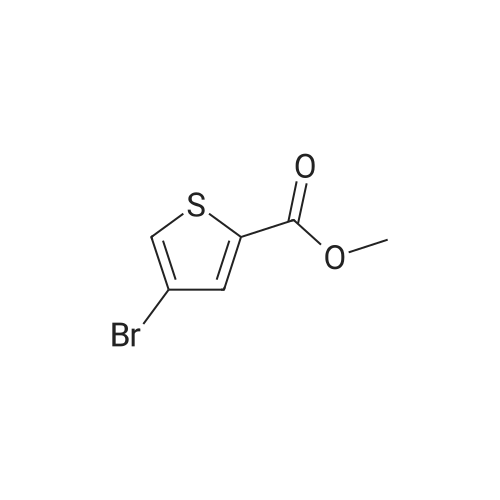
In 100ml single neck flask was added 4-bromo-2-thiophenecarboxylic acid 2.07g (10mmol), thionyl chloride 1.19g (10mmol) and absolute ethanol 25ml, refluxed, 6h After TLC showed the starting material is no longer remaining. The solvent was distilled off under reduced pressure, ice water was added, adjusting the pH with saturated sodium carbonate solution to 9 to 10, and extracted with ethyl acetate (40ml × 3), the combined organic phase was washed twice with saturated brine, dried over anhydrous MgSO 4, pale yellow oily liquid 2.02g, yield 91.4%. |
| 90% |
With sulfuric acid; for 10h;Heating / reflux; |
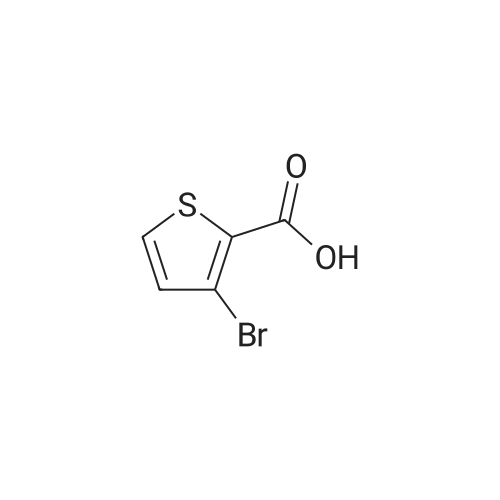
Example 1 : Methyl 4-{6-[4-(2-piperidin-l-ylethoxy)phenyllpyrazolo[l,5-alpyrimdin-3-vUthiophene- 2-carboxylate; Step 1 : methyl 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)thiophene-2-carboxylate; 4-Bromothiophene-2-carboxylic acid (0.418 g, 2 mmol) was dissolved in methanol (1 mL), and concentrated sulfuric acid (0.039 g, 0.4 mmol) was added. The mixture was refluxed for 1O h, poured into water, and subjected to 3-fold extraction with ethyl acetate. The organic layer was washed with K2CO3- solution, concentrated, dried over MgSO4, filtered and evaporated to give methyl 4-bromothiophene-2- carboxylate, weight 0.4 g (90% yield).A flask containing PdCl2(dppf) (0.32 g, 0.43 mmol), dppf (0.24 g, 0.43 mmol), KOAc (4.23 g, 0.043 mol), and pinacolediborone (5.5 g, 0.021 mol) was flushed with argon, then a solution of the ester from the foregoing step (3.2 g, 0.014 mol) in dioxane (60 mL) was added. The mixture was stirred at 850C under argon atmosphere for 40 h. Water (5-fold excess) was added, and the mixture was subjected to 3-fold extraction with ethyl acetate. The organic layer was washed with brine, concentrated, dried over MgSO4, filtered, and evaporated to give the crude methyl 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)thiophene- 2-carboxylate (5.1 g, purity 85% according to 1H NMR data). This crude boronate was used without further purification. |
|
With sulfuric acid;Heating / reflux; |
Step 2 4-Bromo-thiophene-2-carboxylic acid (12.85g), CH30H (360 mL) and H2SO4 (95-98%, 6mL) were refluxed overnight. The solution was basified and evaporated to remove the organic solvent. The residue was extracted with EtOAc. The organic layer was washed with water and brine, then dried over Na2SO4, evaporated to give the product the solvent gave the product 4-Bromo-thiophene-2-carboxylic acid methyl ester (13 g). |
|
With thionyl chloride; at -20℃; for 2.08333h;Reflux; |
4-bromo-2-thiophenecarboxylic acid (3.0 g) was dissolved in anhydrous methanol (30 mL) and cooled to -20 C.Then, thionyl chloride (5.1 g) was slowly added dropwise to the above mixture.After stirring for 5 minutes, the temperature was raised to reflux and the reaction was carried out for 2 h.The reaction was quenched with water and extracted with EtOAc.The extract is washed with saturated brine.After drying, it was concentrated to give a crude material (yellow oil, 3.24 g). |
|
With thionyl chloride; for 6h;Reflux; |
To a stirring solution of 4-bromo-2-thiophenecarboxylic acid (3g, 14.5mmol) in methanol (25mL) was added thionyl chloride (1.74g, 14.5mmol). Reaction mixture was heated to reflux and stirred for 6h. Upon completion, the residue was taken up in ice water (60mL). The pH was adjusted to 9-10 with saturated sodium carbonate solution. The aqueous phase was extracted with ethyl acetate (40mL×3). The combined organic phase was washed twice with saturated brine (20mL×2) and dried over anhydrous MgSO4. After filtering out MgSO4, the solvent of filtrate was removed in vacuo to afford crude product of title compound as a pale-yellow oil which was used for next step directly. Yield: 98%; 1H NMR (300MHz, CDCl3) delta: 7.69 (s, 1H), 7.45 (s, 1H), 3.90 (s, 3H). MS (m/z): [M+H]+ 221.0, 223.0. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping