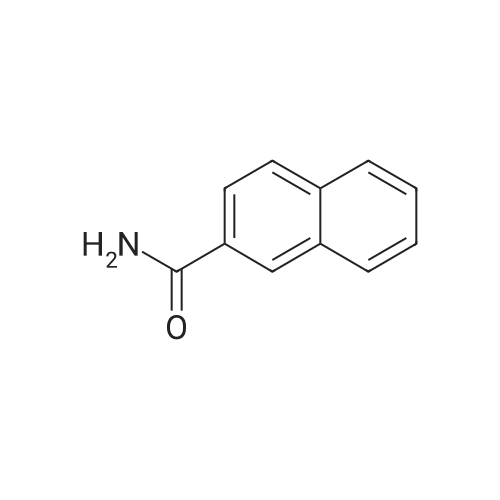
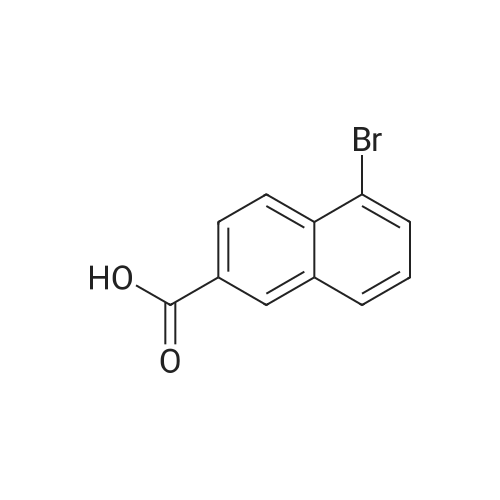
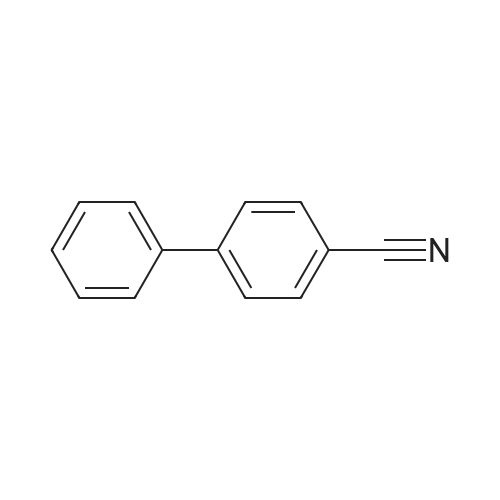
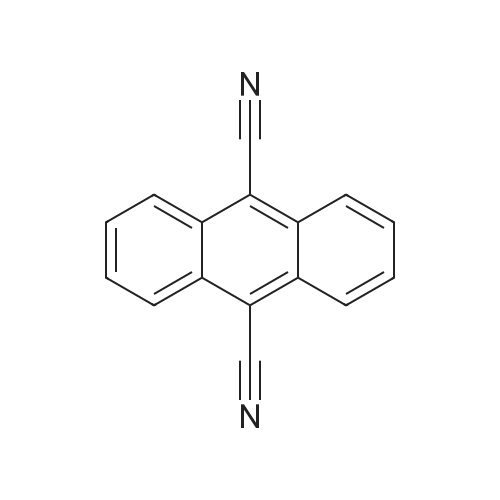
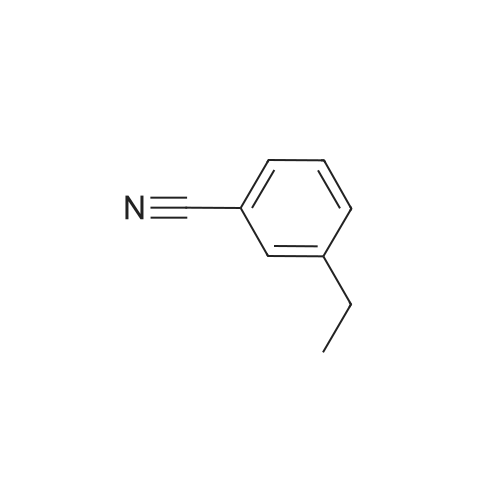
| 96% |
With oxalyl dichloride; triethylamine; In dimethyl sulfoxide; acetonitrile; at 20℃; for 0.666667h;Inert atmosphere; |
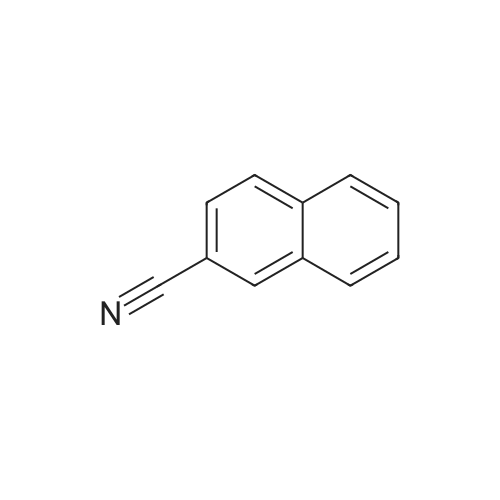
Nitrogen protection, in a 100 mL three-necked flask equipped with a thermometer,Add anhydrous acetonitrile (10 mL) in turn, twoJia Ya satire (0·03mmol, 2·5mg, 0·Olequiv),2-naphthylcarboxamide (3mmol, 513mg, 1 · Oequiv)And triethylamine (1 · 04mL, 7 · 5mmol, 2 · 5equiv),Slowly add oxalyl chloride to the constant pressure dropping funnel at room temperature(0.31 mL, 3.6 mmol, 1.2 equiv) in dry acetonitrile (5 mL).After the addition was completed, stirring was continued for 40 min, suction filtration, and the filtrate was spun dry.Distilled water (15 mL) was added and extracted with ethyl acetate (3 chi 10 mL).The combined organic layers were washed with aq.Filtration, rotary distillation to remove the solvent to obtain a crude product.Purified by column chromatography (petroleum ether / ethyl acetate = 9:1),441 mg of 2-naphthonitrile was obtained in a yield of 96%. |
| 84% |
at 300℃; for 1h; |
General procedure: Following the amide intermediate Preparation Example A. The reaction vessel is closed (when the amide intermediate has a boiling point at normal pressure equal to or lower than the reaction temperature TB described below) or the reaction vessel is kept open (when the amide intermediate has a boiling point higher than the normal pressure When the reaction temperature is TB), the stirring is continued (600 r/min), the reaction temperature is changed to TB, and after the reaction temperature TB is maintained for TD hours, the reaction is almost complete. Then, the reaction vessel was sealed and connected to a vacuum pump so that the degree of vacuum in the reaction vessel reached 20-50 mbar (according to the type of nitrile product) and the distillate was used as the nitrile product. The yield of the nitrile product was calculated and sampled for nuclear magnetic proteomics and elemental analysis to characterize the nitrile product obtained. Specific reaction conditions and characterization results are shown in Tables A-7, A-8, A-9, A-10 and A-11 below. These characterization results show that the nitrile product obtained has an extremely high purity (above 99%).In these nitrile product preparation examples, 10 g of diphosphorus pentoxide was optionally added to the reaction vessel as a catalyst at the start of the reaction. |
| 61% |
With triethylamine; ethanaminium,N-(difluoro-lambda4-sulfanylidene)-N-ethyl-,tetrafluoroborate; In toluene; at 20℃; for 4h;Inert atmosphere; |
General procedure: To a solution of the aldoxime or the amide (1.0 mmol) and Et3N (1.5mmol) in EtOAc (1 mL, 1 M) at r.t. was added XtalFluor-E8 (1.1 mmol)portionwise over ca. 2 min. The resulting solution was stirred at r.t.for 1 h. The reaction mixture was quenched with sat. aq Na2CO3 and extracted with CH2Cl2 (2 × 10 mL). The combined organic layers were washed with H2O and brine, dried (MgSO4), and concentrated under vacuum to afford the crude nitrile, which was purified by flash chromatography, if required. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping