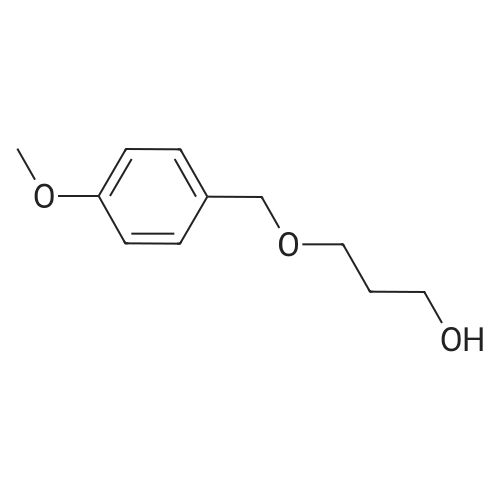
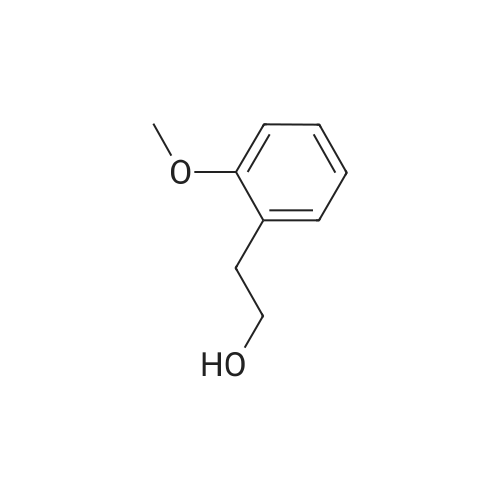
| 93% |
With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; ammonium hydroxide; copper(l) chloride; at 120℃; for 24h; |
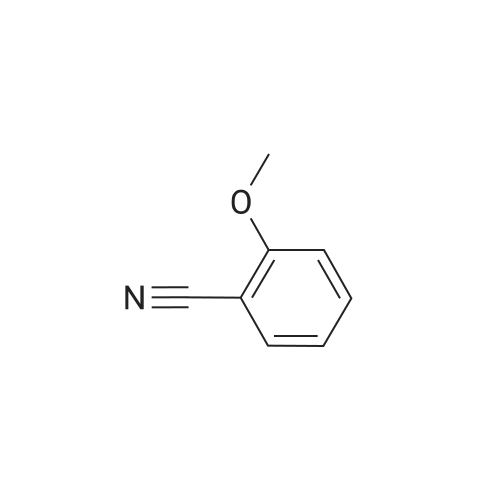
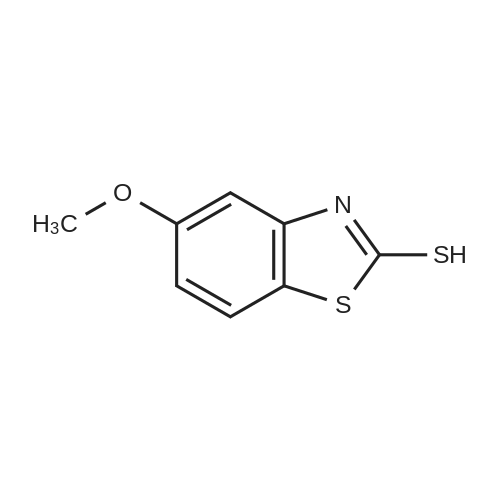
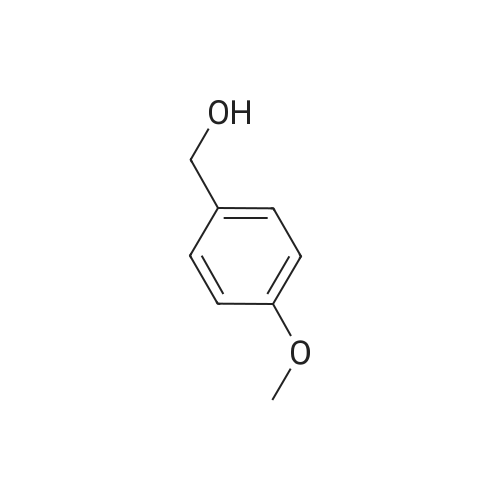
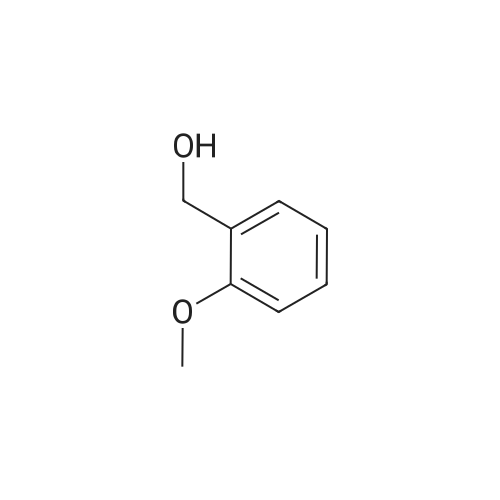
The reactants used were o-methoxybenzyl alcohol (i.e., R1 in the formula (I) was ortho OCH3) 1.0 mmol(138.2 mg), experimental procedure and procedure with Example 1, aqueous ammonia (1.7 mol / L) 5.0 mL,The amount of catalyst used in cuprous chloride was 8 mol% (7.9 mg)TEMPO is used in an amount of 8 mol% (12.5 mg)The reaction temperature was 120 and the reaction time was 24h. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 10: 1) to give the target product as 123.9 mgYield 93%. |
| 90% |
With ammonium hydroxide; copper(l) iodide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-Phenylalanin; sodium hydroxide; In methanol; at 60℃; for 24h;Cooling with ice; |
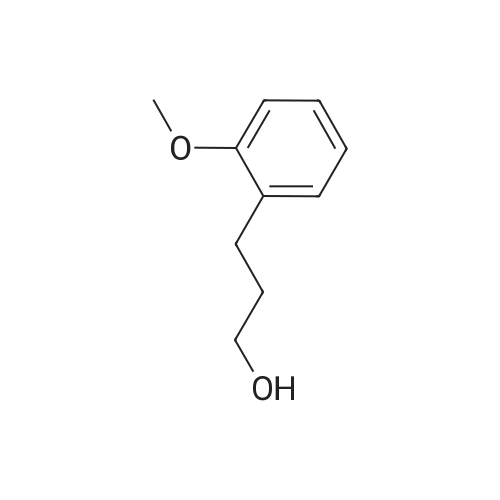
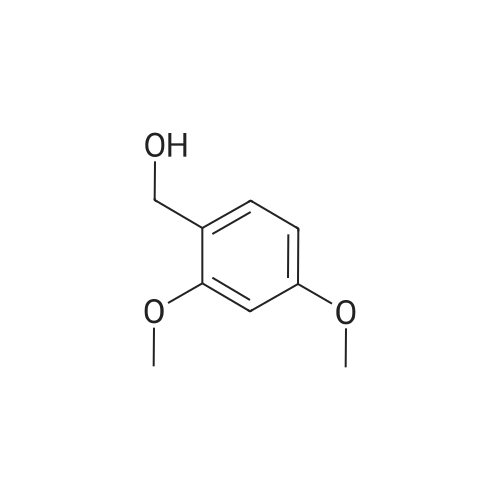
General procedure: Reactants used were p-methylbenzyl alcohol (122.03g, 1000mmol, i.e., of formula (I) wherein R is methyl, n = 1, m = 0 , X = C),cuprous iodide (9.50 g of , 50mmol), N- phenylglycine (7.51g, 50mmol), TEMPO ( 7.80g, 50mmol),sodium hydroxide (4.00g, 100mmol), aqueous ammonia (300mL, 25 ~ 28%) ,ethanol, 800mL, in an ice bath under the condition, with oxygen round bottom flask is evacuated of air ventilation 3 times, and then the system was stirred at 25 , 24h, after completion of the reaction, the reaction solution was cooled to room temperature, rotary evaporated to remove the solvent, the residue was washed with water filtered and dried The product was 107.64g, yield 92%. he reaction was used for the o-methoxybenzyl alcohol (138.02g, 1000mmol, i.e., of formula (I) wherein R is 2-methoxy n = 1, m = 0 , X = C),the same experimental methods and procedures Example 2, except that: copper iodide (9.50g, 50mmol), N- phenylalanine (8.26g, 50mmol), TEMPO ( 7.80g, 50mmol),sodium hydroxide (4.00g, 100mmol ), ammonia (300mL, 25 ~ 28%) ,methanol 800mL, stirred at 60 for 24h, to give the final product 119.71g, yield 90%. |
| 74.7% |
With ammonium hydroxide; manganese sesquioxide; oxygen; at 130℃; under 11251.1 Torr; for 30h;Autoclave; |
General procedure: The ammoxidation of alcohols and hydration of nitrileswere performed in a high-pressure steel autoclave reactorequipped with a PTFE bottle, magnetic stirrer (900 rpm), andan explosion-proof pressure sensor. For the ammoxidation ofalcohols, the as-prepared catalyst, aqueous ammonia(28%-30% NH3), and alcohols were added into a certainamount of t-amyl alcohol solvent in the reactor, then the autoclavewas sealed and purged with oxygen for two times to excludethe inside air. For the hydration of nitrile, the as-preparedcatalyst, nitrile, and water were added into a certain amount oft-amyl alcohol solvent in the reactor, then the autoclave wassealed and purged with N2 for two times to exclude the insideair. After that, the reactor was quickly heated to the desiredtemperature (the reaction temperature was measured by athermocouple in the autoclave) in an oil bath. After a desiredreaction time, the reactor was placed in an ice bath to quenchthe reaction. After separation of the solid catalyst by centrifugation,the liquid was analyzed with a Shimadzu GC-2014 gaschromatograph equipped with a flame ionization detector(FID) and an Agilent HP-6890 gas chromatograph-mass spectrometer,with ethylbenzene, bromobenzene, hexadecane, orbiphenyl used as internal standards. The gas-phase products,such as CO and CO2, were analyzed with a Fu Li-9790 gaschromatograph equipped with a thermal conductivity detector(TCD). Notably, no CO and CO2 signals were observed in TCDand total carbon balances were always >90.0% in this work.Safety Note: The high-pressure oxygen has been extensivelyused in the aerobic oxidations [21,22], and the reaction systemsin this work were out of the explosion limits of the reactants.For example, the explosion limit of benzyl alcohol is1.3%-13.0% in oxygen, and the concentration of benzyl alcoholin the gaseous phase in this work is in a 0-0.4% region, whichis out of the explosion limits. Furthermore, the fire and staticelectricity are not allowed to access the internal reactor forsafety reasons. In the kinetics study, the average reaction rateswere calculated from the moles of substrate converted pergram of catalyst in one hour (mmol gcat-1 h-1), with the conversionof substrate controlled to be lower than 20.0%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping