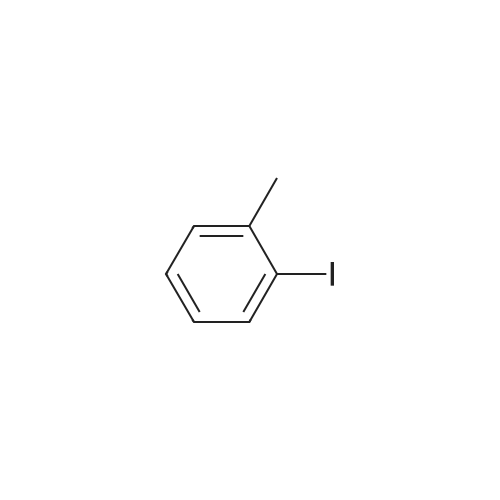
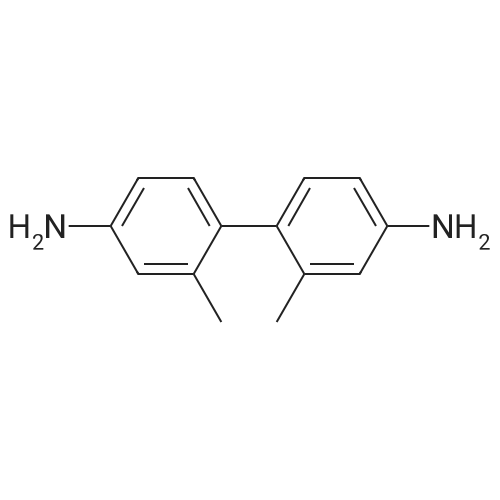
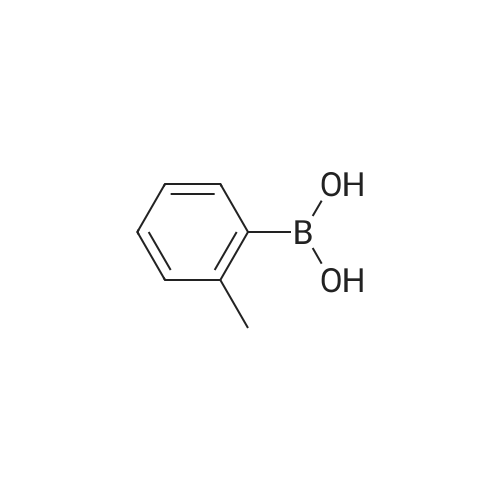
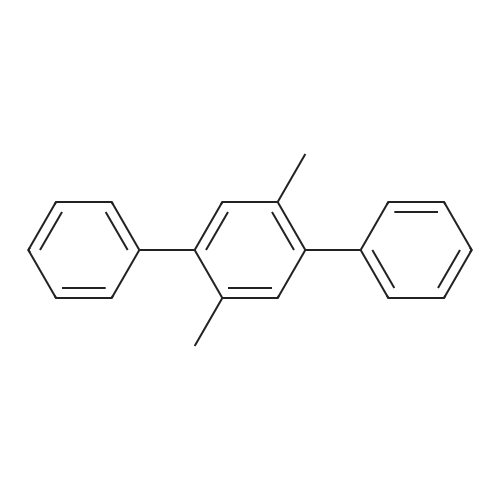
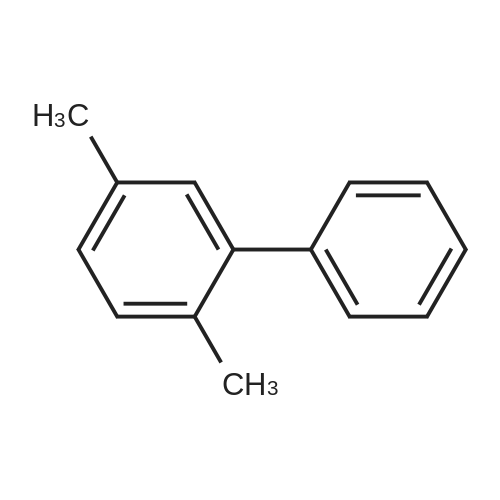
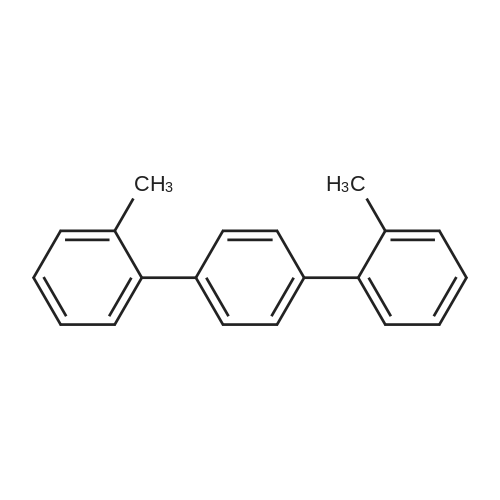
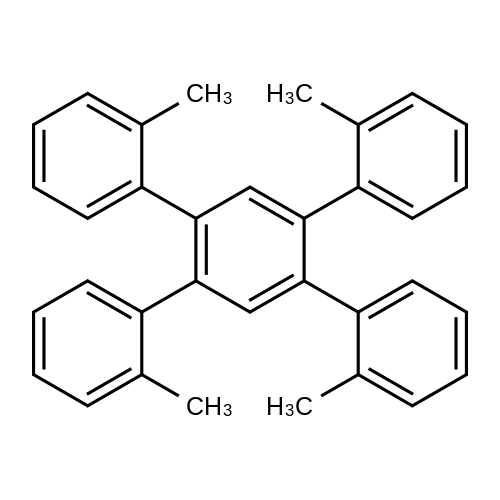
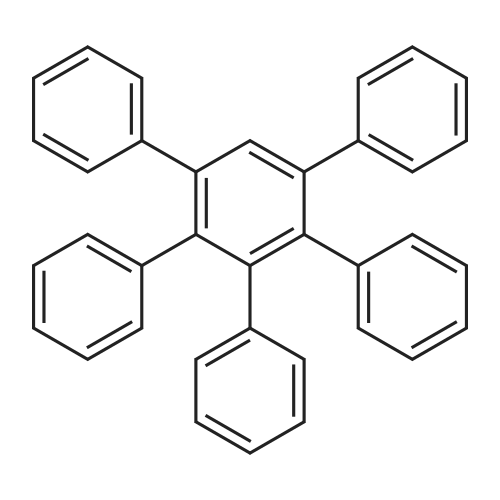
| 78% |
With tetrabutylammonium nitrate; sodium hydroxide; palladium dichloride; at 120℃; for 7h;Catalytic behavior; |
General procedure: Aryl halide (2.0 mmol) was added to a flask containingtetrabutylammonium nitrate (0.5 mmol, 0.15 g), PdCl2(0.03 mmol, 0.0053 g), and NaOH (2.0 mmol, 0.08 g),and the mixture was stirred at 120 C. After completion ofthe reaction, which was detected by TLC, the mixture wascooled to room temperature and the coupled product wasextracted with diethyl ether (3 × 3 mL). The solvent wasthen evaporated to leave the crude product, which was purifiedby column chromatography over silica gel using n-hexaneas the eluent to give the pure product. |
| 65% |
With triethylamine; In neat (no solvent); at 100℃; for 5h;Sealed tube; Irradiation; |
General procedure: In a typical reaction, a mixture of aryl halid (0.25mmol),Et3N(0.25mmol) and TiO2-AA-Pd nanohybrid (0.3mol%)was added in a 10mL Pyrex test tube and sealed with septumcap. Then the reaction mixture transferred into a reactor chamber and irradiated under magnetic stirring using a CFL lamp (philips, wavelength in the range 390-750nm, 40W,1.1Wm-2) as the visible light source at 100C for appropriate time. After completion of the reaction, TiO2-AA-Pd nanohybrid was extracted by adding of ethanol (5ml) followedby centrifuging and decantation (3 × 5mL ethanol).Then, desired product (liquid phase) was extracted by platechromatography eluted with n-hexane/EtOAc (10/2). |
| 28% |
With triethylamine; In neat (no solvent); at 110℃; for 10h;Green chemistry; |
General procedure: A mixture of aryl halide (0.125mmol), NEt3(0.375mmol)and catalyst (0.001g, 0.03mol%) was stirred at 110C forthe appropriate of time. The progress of the reaction was monitored by TLC. After completion of the reaction, Pd(II)NA2SMNP nanohybrid was extracted by adding of ethanol(5ml) followed by washing and decantation in the presenceof an external magnet (3 × 5ml ethanol). Then, desired product(liquid phase) was extracted by plate chromatographyeluted with n-hexane/EtOAc (10/1). |
| 71.9%Chromat. |
With porous chitosan microspheres supported palladium catalyst; In ethylene glycol; dimethyl sulfoxide; at 110℃; for 23h; |
General procedure: To a 20 ml round bottom flask containing 5.0 ml of solvent, added aromatic halide (1.0 mmol), palladium catalyst (0.02 mmol) and potassium acetate (7.5 mmol). The resulting mixture was allowed to stir and the reaction progress was monitored by TLC and/or GC/MS analysis. The reaction was quenched with 10 ml water after completion and the mixture was extracted with ethyl acetate (3 × 20 ml). The combined organic extract was washed with water, saturated brine, and then dried with anhydrous Na2SO4. Solvent was removed under a reduced pressure, and the coupling product was purified by silica gel chromatography with petroleum ether and ethyl acetate. The homocoupling products are known and they are all consistent with the related chemical structures as characterized from 1H NMR and GC/MS analysis. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


 complexes Spectroscopic evidence, rates, and selectivity.png)