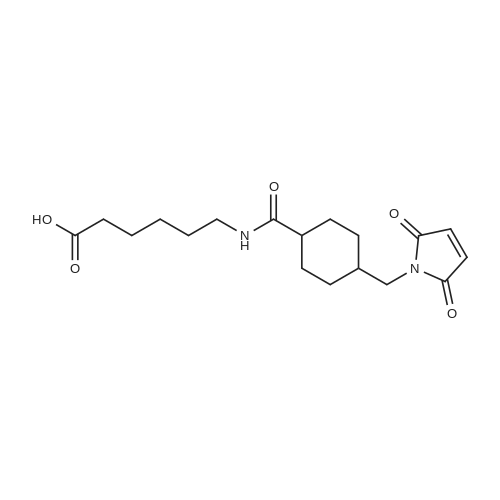
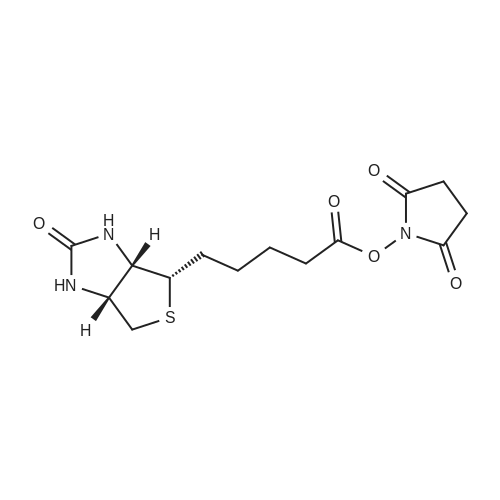
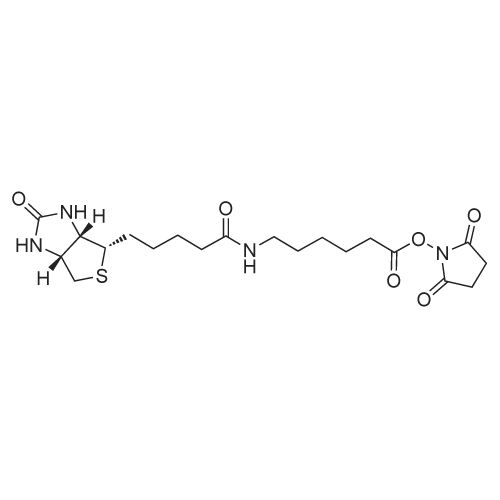
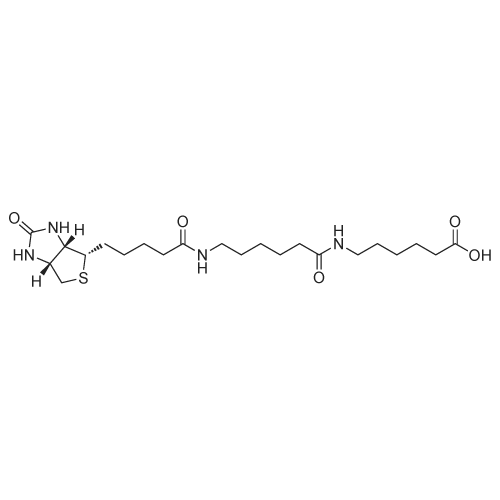
| 75% |
In N,N-dimethyl-formamide; at 20℃; |
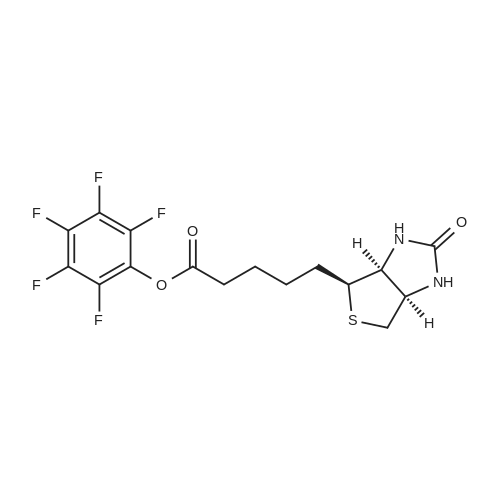
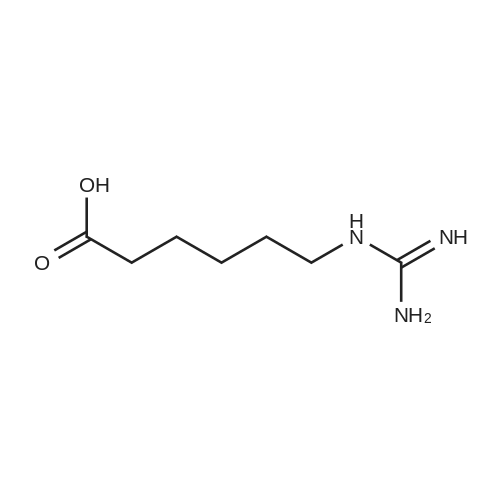
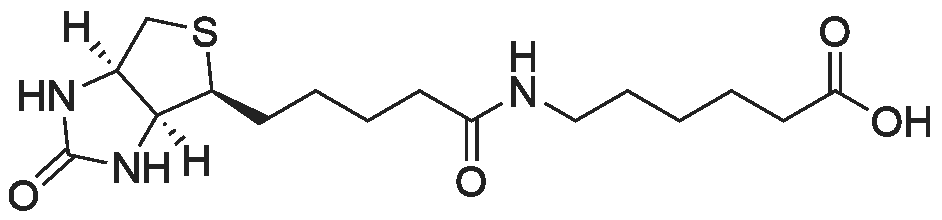
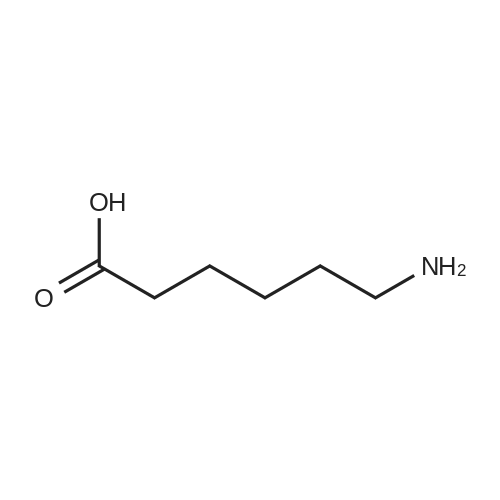
biotin-NHS (1 eq., 388 mg, 1.14 mmol) was dissolved in the anhydrous DMF (8.54 mL). To the resulting solution was added 6-aminocaproic acid (1 eq., 149 mg, 1.14 mmol) andthe mixture was stirred at r.t. overnight and then concentrated to yield 1:1 mixture of the product and NHS. To the product was then added 95:1 :4 mixture of EtOH-AcOH-H20. The resulting suspension was heated to boiling, filtered, and left to crystallize to yield compound 9 (305 mg, 0.854 mmol, 75 percent) as a white fluffy solid. The structure of 9 was confirmed by ESI-MS analysis (Method 1).ESI-MS m/z: 358.3 [M+H] |
| 1.455 g |
With sodium hydrogencarbonate; In water; N,N-dimethyl-formamide; at 20℃; for 16h; |
epsilon-Aminocaproic acid (966mg, 8.4mmol) was dissolved in 30mL 1M aqueous NaHCO3 solution. Then N-hydroxysuccinimidobiotin (3.09g) in 35mL DMF was added dropwise to the solution and the mixture was stirred at room temperature for 16h. The solution was concentrated under reduced pressure to remove partial solvent and 150mL aqueous citric acid (100g/L) was then added and stirred at 60°C for 30min. The precipitate was collected by filtration and washed with distilled water. The precipitate was dissolved in a mixture of isopropanol and water (8:2, v/v) and kept at 4°C to give pure 3 (1.455g, 50percent) as a yellowish crystal; IR cm?1 (KBr): 3200?3500, 3070, 2932, 2859, 1696, 1644, 1543, 1475, 1391, 1324, 1265, 1118; 1H NMR (600MHz, DMSO-d6): delta 7.75 (1H, t, J=5.4Hz, C6?NH), 6.45 (1H, s, C13?NH), 6.38 (1H, s, C14?NH), 4.35?4.27 (1H, m, H-14), 4.12 (1H, m, H-13), 3.09 (1H, m, H-12), 3.00 (1H, m, H-6) 2.82 (1H, dd, J=12.4, 5.1Hz, H-15a), 2.57 (1H, d, J=12.4Hz, H-15b), 2.19 (2H, t, J=7.4Hz, H-2), 2.04 (2H, t, J=7.3Hz, H-8), 1.20?1.67 (14H, m). 13C NMR (150MHz, DMSO-d6): delta 171.84 (C-1, C-7), 162.74 (C-16), 61.06 (C-13), 59.20 (C-14), 55.44 (C-12), 39.68 (C-15), 38.25 (C-6), 35.21 (C-8), 29.05 (C-2), 28.92, 28.21, 28.04, 26.04, 25.73, 25.36. |
| 368.3 mg |
With sodium hydrogencarbonate; In water; acetone; at 20℃; for 12h; |
General procedure: Biotin-NHS was ammonolyzed withlinkers to afford biotin-linker products [Biotin-AAn-COOH (n = 1, 3, 5, and 7) orBiotin-PEG(n+1)-COOH (n = 1, 2 and 3)]. The linker [AAn (n = 1, 3, 5, and 7) orNH2-PEG(n+1)-COOH (n = 1, 2 and 3), 1 eq.] was dissolved in 4 mL water solutionrespectively, and 240.0 mg NaHCO3 was added to the solution. The reaction mixturewas stirred and the Biotin-NHS (1 eq.) and acetone (500 ul) were added subsequently.After stirring for 12h at room temperature, the solvent was removed by vacuum, andthe crude was dispersed in water, adjusting the pH by 2M HCl to pH=3. The mixturesolution was filtered, washed by water and concentrated under reduced pressure toachieved Biotin-linker products [Biotin-AAn-COOH (n = 1, 3, 5, and 7) orBiotin-PEG(n+1)-COOH (n = 1, 2 and 3)]2, 3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping