|
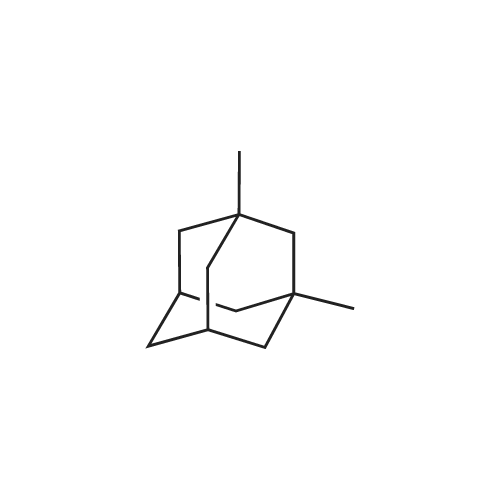
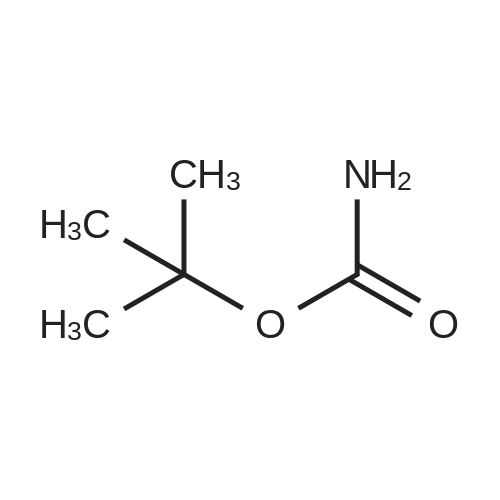
dibutyldimethoxytin; In Triethylene glycol dimethyl ether; at 111.657 - 161.101℃; for 22h;Compresed liquid(s);Conversion of starting material; |
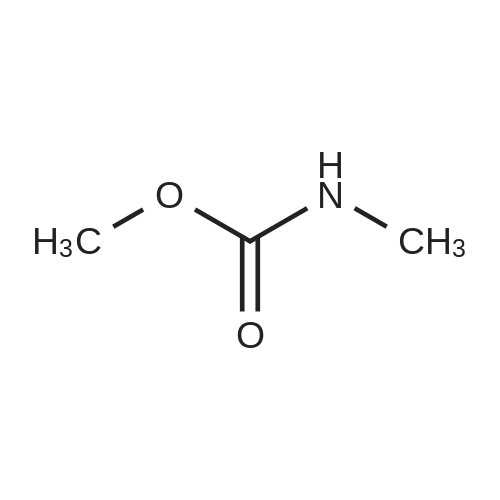
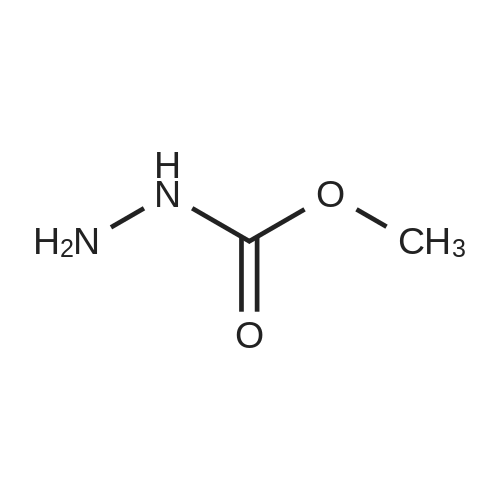
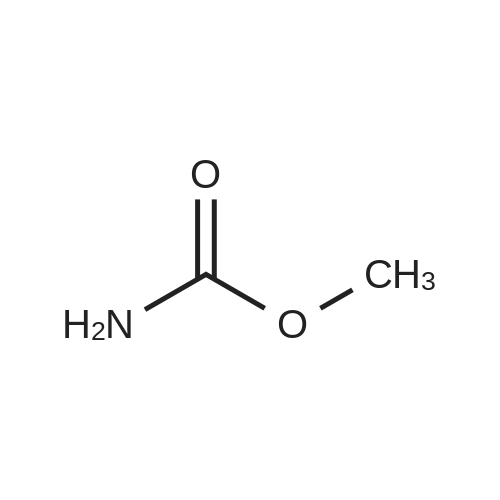
EXAMPLE 3 This Example illustrates the actual production of DMC by one step. The reboiler of the distillation still was charged with 125 g methyl carbamate, 120 g methanol, 80 g triglyme and 25 dibutyltin dimethoxide. The reboiler temperature was maintained at 349-357 F. by controlling the overhead pressure during the 12 hours uninterrupted run. The flow rate of the overhead liquid product was set at 2 cc/min. A urea solution prepared by dissolving 105.6 g urea in 2200 g methanol was pumped into the reboiler to maintain a constant liquid level in the reboiler. The reaction was terminated after 12 hours uninterrupted operation. The result of this experiment is listed in Table 2. The change of the DMC composition in the overhead products is shown in FIG. 3. The overhead pressures at the beginning and the end of 12 hours run were 66 and 134.7 psig, respectively. The column temperatures at the bottom and top section of the column were 248 F. and 233 F. at the beginning, and 286 F. and 274 F. at the end of 12 hours, respectively. While the analysis of the sample taken from the reboiler a the end of 12 hours run indicated 3.8% dimethyl carbonate, 20.9% methanol, 21.1% methyl carbamate, 1.5% N-MMC, 52.0% triglyme, 0.2% unknown, 0.2% methylamine (or water) and 0.3% ammonia, the overhead product contained 9.0% dimethyl carbonate, 88.4% methanol, 0.1% methylamine (or water) and 2.5% ammonia. The content of urea in the bottom product sample was unknown because urea could not be analyzed by gc due to urea decomposition. The unit was shut down for the next day's run. The weight of the composite overhead product was 1054 g and the weight of the urea solution pumped into the reboiler was 1252 g. The total samples taken out from the unit was 210.8 g. There was lower liquid level in the reboiler from 8 to 12 hours on stream. The composite overhead product contained 11.5% dimethyl carbonate. A vent gas was collected for 12 hours during the reaction (very little gas volume) and the analysis of this vent gas indicated 0.05 vol % CO2 and 2.1 vol O2 indicating very little decomposition of methyl carbamate or urea. Samples of the overhead product were taken hourly over the duration of the run. The DMC concentration in these samples is illustrated in FIG. 3. The result of this experiment is summarized in Table 2. The maximum concentration of DMC was -16 wt % in the 5 hr sample. The productivity observed at 5 hr was assumed to be indicative of the space yield that could be achieved with the system under steady state conditions. The value was calculated to be -3.4 lb DMC/hr-ft3 (2 g/min×0.16/350 cm2×60 min/hr×2.2E-0.3 lb/g×2.832E+04 cm3/ft3). This value was used in sizing the reaction zone of the reactive distillation column. The run was continued the next day by pumping a mixed solution prepared by mixing 1650 g methanol with 142.5 g triglyme into the reboiler. The reboiler temperature was maintained at 348-359 F. by controlling the overhead pressure. The flow rate of the overhead liquid product was set a 2 cc/min. The reaction was terminated after 10 hours uninterrupted operation. The result of this experiment is listed in Table 2. The overhead pressures at the beginning and the end of 10 hours uninterrupted run were 232.1 and 201.7 psig, respectively. The column temperatures at the bottom and top section of the column were 248 F. and 233 F. at the beginning, and 322 F. and 313 F. at the end of 10 hours run, respectively. While the analysis of the sample taken from the reboiler at the end of 10 hours (total 22 hours from the very beginning) run indicated 1.7% dimethyl carbonate, 22.2% methanol, 1.5% methyl carbamate, 1.3% N-MMC, 71.9% triglyme, 1.3% unknowns and 0.1% air, the overhead product contained 3.8% dimethyl carbonate, 94.94% methanol and 1.2% ammonia. The content of urea in the bottom product sample was unknown, because urea could not be analyzed by gc due to urea decomposition. The weight of the composite overhead product was 956 g and the weight of the mixed solution pumped into the reboiler was 1088. The total weight of the samples taken out from the unit was 197.2 g. The total weight of the inventory material collected from the column and the reboiler was 249. The vent gas was collected during the run (very small gas volume) and it contained 10.0 vol% CO2 and 0.7 vol O2. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping