| 91.8 mmol, 67% |
With hydrogenchloride; sulfuric acid In methanol |
Step C:
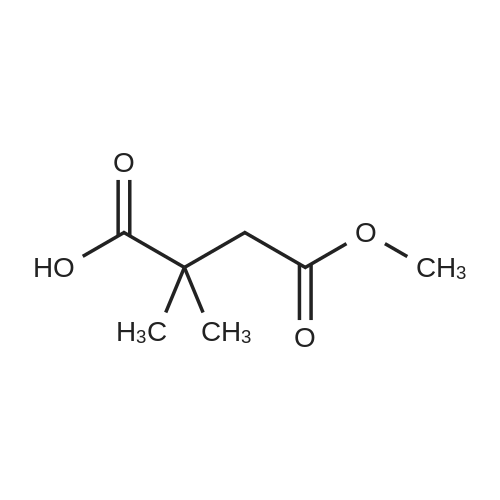
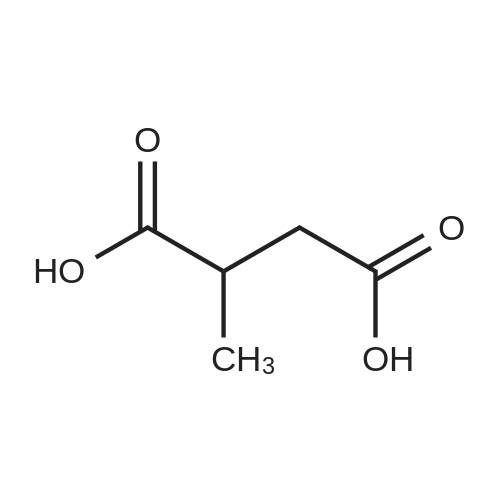
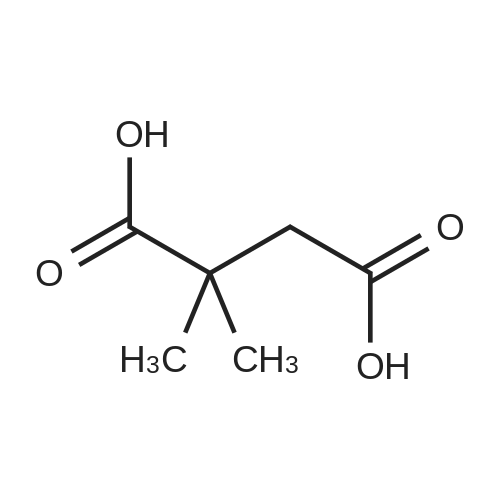
2,2-Dimethylbutanedioic acid, 4-methyl ester
2,2-dimethylsuccinic acid (20 g, 137 mmol) dissolved in 200 ml absolute methanol at 0° was treated dropwise with 2 mL concentrated sulfuric acid.
After the addition was complete, the mixture was allowed to warm to room temperature and stirred for 16 hours.
The mixture was concentrated in vacuo to 50 mL and slowly treated with 200 mL of saturated aqueous sodium bicarbonate.
The mixture was washed with hexane (3*) and the aqueous layer removed and cooled in an ice bath.
The mixture was acidified to pH 2 by slow addition of 6N HCl then extracted with ether (8*).
The combined extracts were washed with brine, dried over magnesium sulfate, filtered and solvents removed in vacuo.
The residue was dried at room temperature under vacuum to afford 14.7 g (91.8 mmol, 67percent) of a viscous oil that slowly solidified upon standing.
1 H NMR analysis indicates the product is a mixture of the title compound and 15percent of the isomeric 2,2-dimethylbutanedioic acid, 1-methyl ester. NMR (200 MHz, CDCl3) of title compound: 1.29 (s,6H), 2.60 (s,2H), 3.66 (s,3H). NMR (200 MHz, CDCl3) of isomer: 1.28 (s,6H), 2.63 (s,2H), 3.68 (s,3H).
|
| 91.8 mmol, 67% |
With hydrogenchloride; sulfuric acid In methanol |
Step A
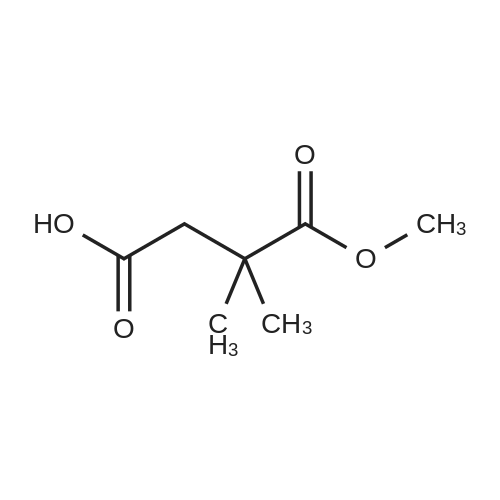
2,2-Dimethylbutanedioic acid, 4-methyl ester
2,2-Dimethylsuccinic acid (20 g, 140 mmol) dissolved in 200 mL of absolute methanol at 0° was treated dropwise with 2 mL of concentrated sulfuric acid.
After the addition was complete, the mixture was allowed to warm to room temperature and stirred for 16 hours.
The mixture was concentrated under vacuum to 50 mL and slowly treated with 200 mL of saturated aqueous sodium bicarbonate.
The mixture was washed with hexane (3*) and the aqueous layer removed and cooled in an ice bath.
The mixture was acidified to pH 2 by slow addition of 6 N HCl then extracted with ether (8*).
The combined extracts were washed with brine, dried over magnesium sulfate, filtered and solvents removed under vacuum.
The residue was dried at room temperature under vacuum to afford 14.7 g (91.8 mmol, 67percent) of a viscous oil that slowly solidified upon standing.
|
| 91.8 mmol, 67% |
With hydrogenchloride; sulfuric acid In methanol |
Step J
2,2-Dimethylbutanedioic acid, 4-methyl ester
2,2-Dimethylsuccinic acid (20 g, 137 mmol) dissolved in 200 mL of absolute methanol at 0° C. was treated dropwise with 2 mL of concentrated sulfuric acid.
After the addition was complete, the mixture was allowed to warm to room temperature and stir for 16 hours.
The mixture was concentrated under vacuum to 50 mL and slowly treated with 200 mL of saturated aqueous sodium bicarbonate.
The mixture was washed with hexane (3*) and the aqueous layer removed and cooled in an ice bath.
The mixture was acidified to pH 2 by slow addition of 6N HCl then extracted with ether (8*).
The combined extracts were washed with brine, dried over magnesium sulfate, filtered and solvents removed under vacuum.
The residue was dried at room temperature under vacuum to afford 14.7 g (91.8 mmol, 67percent) of the product as a viscous oil that slowly solidified upon standing. 1 H NMR (200 MHz, CDCl3): 1.29 (s, 6H), 2.60 (s, 2H), 3.65 (s, 3H).
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping