| 89% |
|
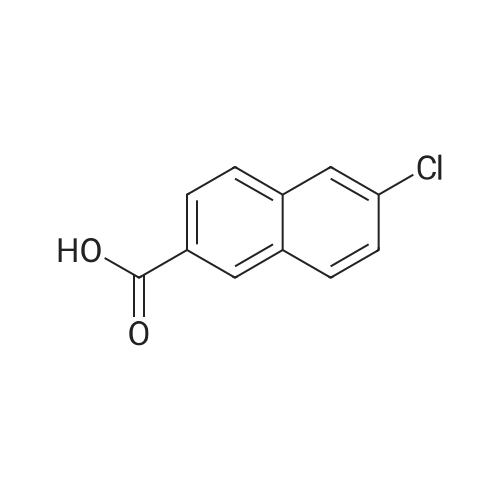
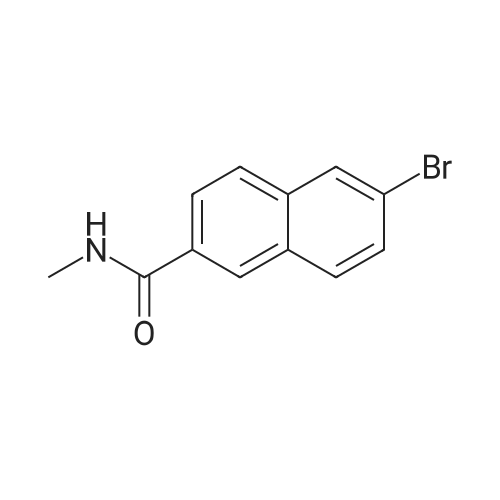
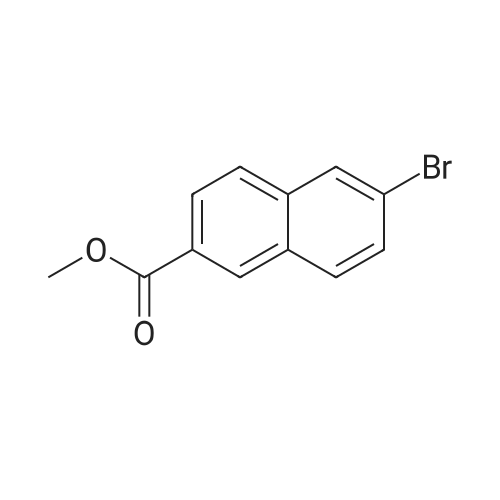
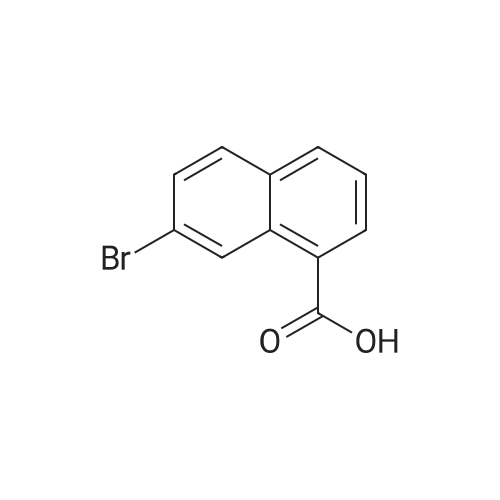
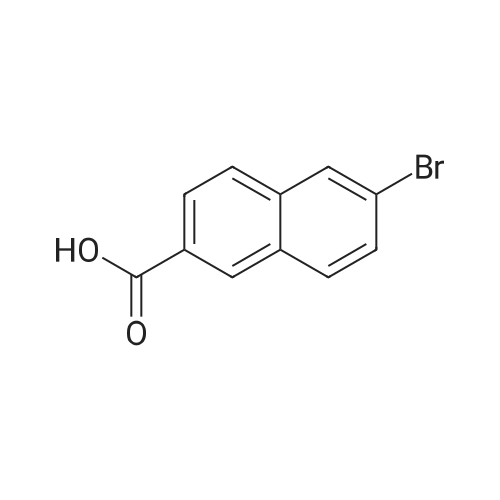
Reference Example 76-Bromo-2-naphthoic acid (10.1 g, 40.1 mmol) and N,N- dimethylformamide (4.75 g, 65.0 mmol) were added to toluene (80 mL) . To the reaction mixture was added dropwise thionyl chloride (5.7 g, 48.2 mmol) at 45 to 50C, and the mixture was stirred for 1 hr, and allowed to cool to room temperature. The reaction mixture was added dropwise at 10 to 25C to a solution prepared by adding triethylamine (11.4 g, 112.4 mmol) and 40% methylamine methanol solution (8.1 g, 104.4 mmol) to toluene (80 rnL) , and the mixture was stirred at room temperature for 1 hr.. To the reaction mixture was added dropwise water (50 mL) , and the mixture was stirred at room temperature. The crystals were collected by filtration, and washed with a mixed solvent (25 mL) of methanol/water (2:8) to give wet crystals. The total amount of the wet crystals was added to N,N- dimethylacetamide (70 mL) , and dissolved with heating to 60C. The reaction mixture was allowed to cool to room temperature, and water (140 mL) was added dropwise thereto. The crystals were collected by filtration, and washed with water (80 mL) to give wet crystals. The total amount of the wet crystals was suspended in ethyl acetate -(25 mL) with stirring at roomtemperature. The crystals were collected by filtration, and washed with ethyl acetate (5 mL) . The obtained wet crystals were dried under reduced pressure to give 6-bromo-N-methyl-2- naphthamide (9.4 g, 35.6 mmol). yield 89%.? NMR (500 MHz, DMSO-d6) delta 2.84 (d, J = 4.4 Hz, 3H) , 7.71 (dd, J = 8.8, 2.2 Hz, 1H) , 7.93 - 8.03 (m, 3H) , 8.28 (d, J = 1.9 Hz, 1H), 8.44 (s, 1H) , 8.62 (d, J = 4.1 Hz, 1H) ; HRMS (ESI) m/z Calcd for a Ci2HuNOBr [M+H]+: 264.0024, Found: 264.0019; Anal. Calcd for a Ci2Hi0NOBr: C, 54.57; H, 3.82; N, 5.30; Br, 30.25. Found: C, 54.56; H, 3.70; N, 5.34; Br, 30.23. |
| 82% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; N,N-dimethyl-formamide; at 0 - 20℃; for 18h;Inert atmosphere; |
Under an argon atmosphere, to a cooled (0 C) solution of 8 (60.26 g, 240 mmol), EDCI·HCl (55.21 g, 288 mmol), HOBt·H2O (44.1 g, 288 mmol) and N,N-diisopropylethylamine ((i-Pr)2NEt) (37.23 g, 288 mmol) in anhydrous N,N-dimethylformamide (DMF) (960 mL) was added dropwise to a solution of MeNH2 (2 M solution in THF; 192 mL, 384 mmol) and the whole was stirred at room temperature for 18 h. After dilution with water, the precipitate was filtered off, washed with H2O and i-Pr2O and dried under the reduced pressure to give 9a (60.6 g, 82%) as a colorless powder. 1H NMR (CDCl3 + CD3OD) delta: 3.04 (3H, s), 7.60 (1H, dd, J = 1.8 Hz, 8.6 Hz), 7.78 (2H, d, J = 8.6 Hz), 7.85 (1H, dd, J = 1.8 Hz, 8.6 Hz), 8.03 (1H, d, J = 1.8 Hz), 8.25 (1H, s). IR (KBr): 3274, 1638, 1622, 1559, 1495, 1408, 1316, 1159 cm-1. Anal. Calcd for C12H10NOBr.0.1H2O: C, 54.20; H, 3.87; N, 5.27; Br, 30.25. Found: C, 54.03; H, 3.72; N, 5.24. |
| 80% |
|
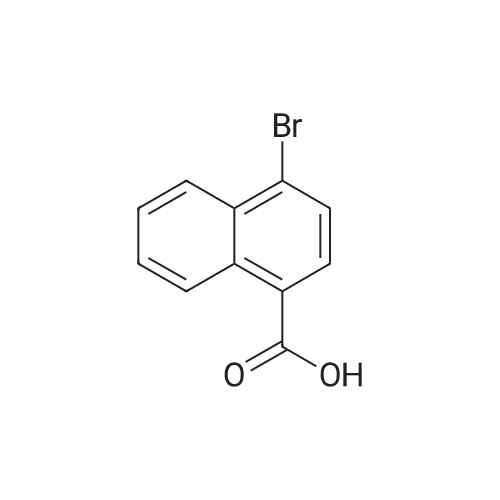
4 Liters of ethyl acetate and 25 ML of DMF were added to 500 g (1.99 mol) of 6-bromo-2-naphthoic acid. 188 ML (2.61 mol, 1.3eq) of thionyl chloride was added dropwise at 30C or lower.. The mixture was stirred at 65C for 30 minutes.. After cooled to 25C, a mixture of 408 ML (3.93 mol, 2eq) of a 40% solution of methylamine in methanol and 558 ML (4.01 mol, 2eq) of triethylamine was added dropwise at 25C or lower.. The mixture was stirred at 25C for 3 hours. 2.5 Liters of water was added dropwise at 25C or lower.. Crystals were filtered, and washed successively with 1.25 liters of a mixed solution of methanol/water=1/4.. Vacuum drying (50C) to a constant weight afforded 422 g of 6-bromo-N-methyl-2-naphthamide (yield 80%).1H NMR (CDCl3+CD3OD): delta 3.04 (3H, s), 7.60 (1H, dd, J=8.6, 1.8 Hz), 7.78 (2H, d, J=8.6 Hz), 7.85 (1H, dd, J=8.6, 1.8 Hz), 8.03 (1H, d, J=1.8 Hz), 8.25 (1H, s). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping