| 76% |
With tetra-n-butylammonium tribromide; sodium hydrogencarbonate; sodium chloride; In dichloromethane; water; |
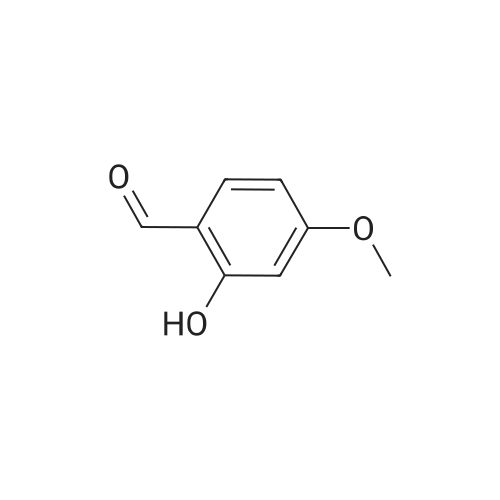
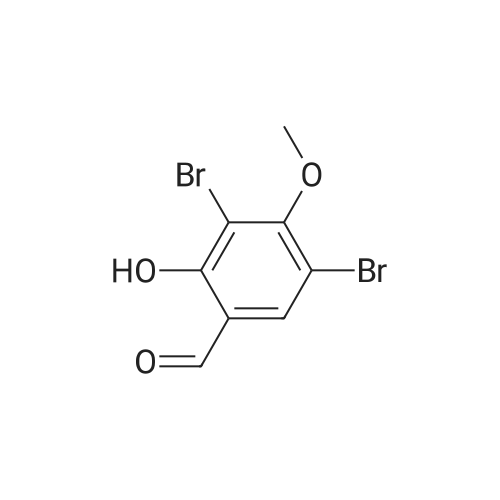
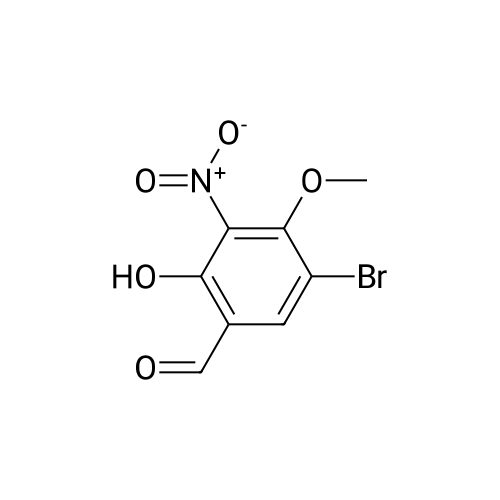
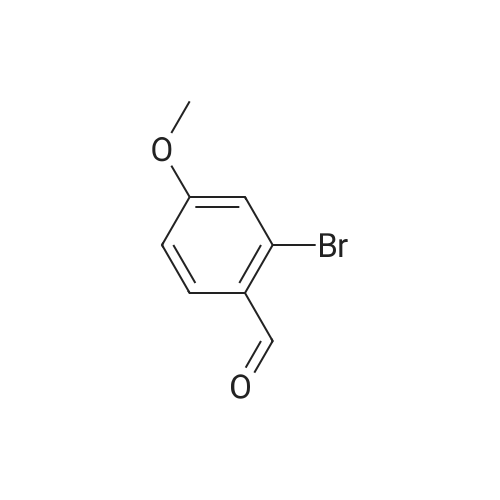
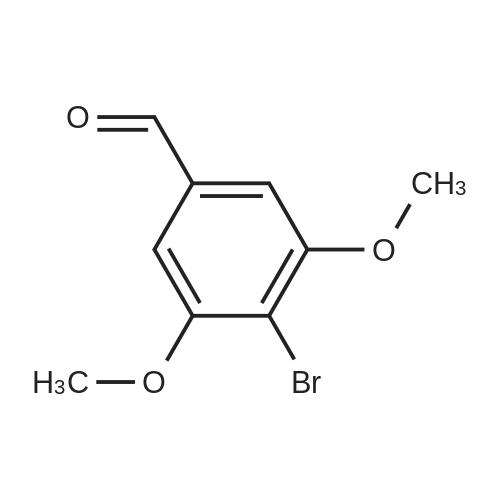
a. 3-Bromo-6-hydroxy-4-methoxy-benzaldehyde. A mixture of 2-hydroxy-4-methoxy-benzaldehyde (3.04 g, 20 mmol) and tetrabutylammonium tribromide (6.40 g, 20 mmol) in anhydrous dichloromethane (200 mL) was stirred at room temperature for 24 hours. The solution was washed successively with a saturated aqueous solution of NaHCO3 (150 mL), water (150 mL), a saturated aqueous solution of NaCl (150 mL), dried over MgSO4 and filtered. Removal of the solvent under reduced pressure gave a solid which was purified by column chromatography, using a Biotage 40M cartridge, eluding with 5% ethyl acetate/95% hexane to give 3-bromo-6-hydroxy-4-methoxy-benzaldehyde as a white solid (3.50 g, 76%). 1H NMR (500 MHz; CDCl3): delta3.94 (s, 3 H), 6.47 (s, 1 H), 7.67 (s, 1 H), 9.68 (s, 1 H), 11.43 (s, 1 H). |
| 76% |
With tetra-n-butylammonium tribromide; sodium hydrogencarbonate; sodium chloride; In dichloromethane; water; |
a. 3-Bromo-6-hydroxy-4-methoxy-benzaldehyde A mixture of 2-hydroxy-4-methoxy-benzaldehyde (3.04 g, 20 mmol) and tetrabutylammonium tribromide (6.40 g, 20 mmol) in anhydrous dichloromethane (200 mL) was stirred at room temperature for 24 hours. The solution was washed successively with a saturated aqueous solution of NaHCO3 (150 mL), water (150 mL), a saturated aqueous solution of NaCl (150 mL), dried over MgSO4 and filtered. Removal of the solvent under reduced pressure gave a solid which was purified by column chromatography, using a Biotage 40M cartridge, eluding with 5% ethyl acetate/95% hexane to give 3-bromo-6-hydroxy-4-methoxy-benzaldehyde as a white solid (3.50 g, 76%). 1H NMR (500 MHz; CDCl3): delta3.94 (s, 3H), 6.47 (s, 1H), 7.67 (s, 1H), 9.68 (s, 1H), 11.43 (s, 1H). |
|
With bromine; In dichloromethane; ethyl acetate; |
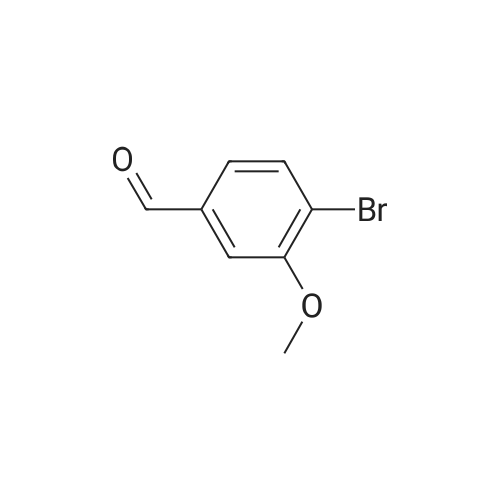
Example 62 3-[5-(Benzo[b]thien-2-yl)-2-carboxymethoxy-4-methoxyphenyl]-1-(3,4,5-trimethoxyphenyl)-2-propen-1-one, Sodium Salt Ex-62A: A solution of 2-hydroxy-4-methoxybenzaldehyde (3.03 g, 20 mmol) in 25 mL of dichloromethane was cooled to 0 C. and treated dropwise with a solution of bromine (3.41 g, 21 mmol) in 10 mL of dichloromethane. The reaction mixture was stirred at 0 C. for 1.5 hours. The solvent was removed by rotary evaporation to give a residue. The residue was taken up in EtOAc and washed with 3 portions of water. The organic layer was dried over MgSO4. The drying agent was removed by filtration, and solvent was removed by rotary evaporation to give 3.9 g of the desired 5-bromo-2-hydroxy-4-methoxybenzaldehyde as a solid, m.p. 111-115 C. |
|
With bromine; |
2-Hydroxy-4-methoxybenzaldehyde (20 g, 131.4 mmol) was brominated to give 5-bromo-2-hydroxy-4-methoxybenzaldehyde as a light yellow solid, followed by coupling with 4-methoxyphenylboronic acid (750 mg, 4.94 mmol) using method A to give 800 mg (75%) of 4-hydroxy-6,4'-dimethoxybiphenyl-3-carbaldehyde as a light yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping