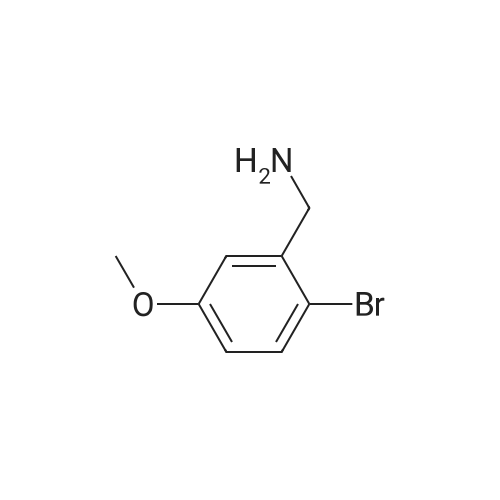
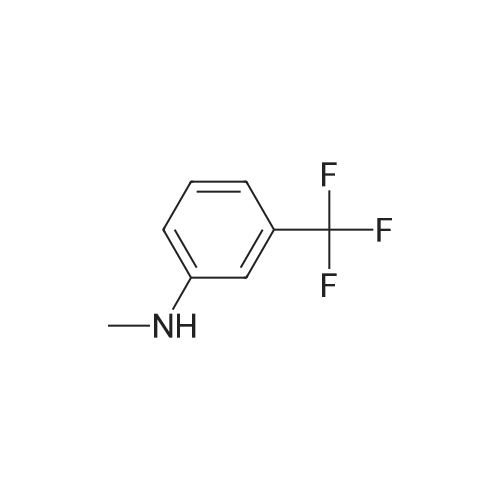
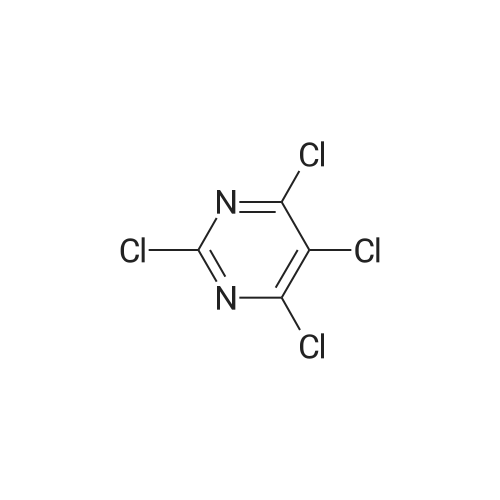
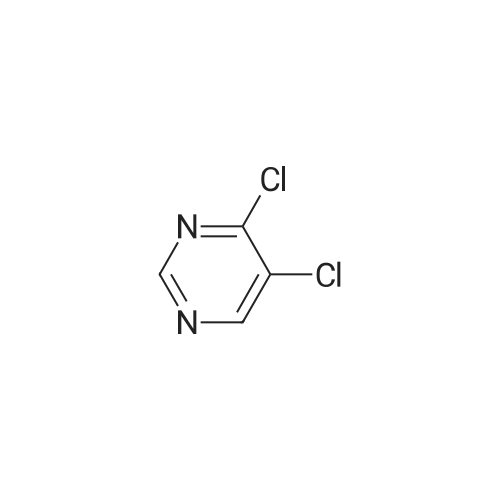
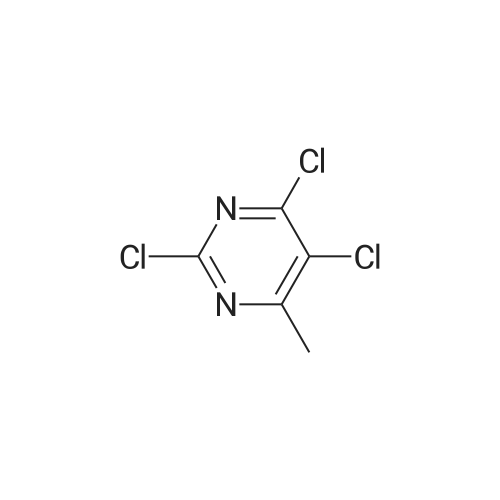
| 61% |
With potassium carbonate; In N,N-dimethyl-formamide; at 60℃; for 5.0h; |
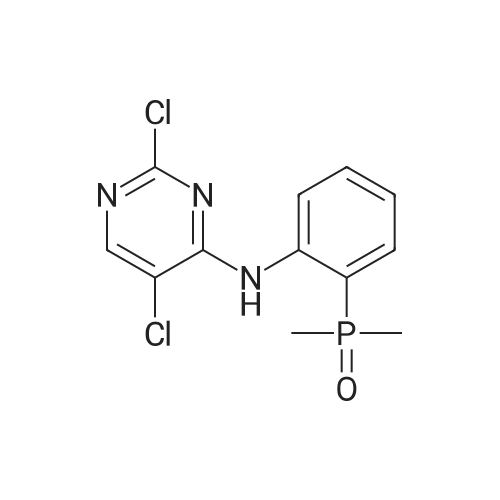
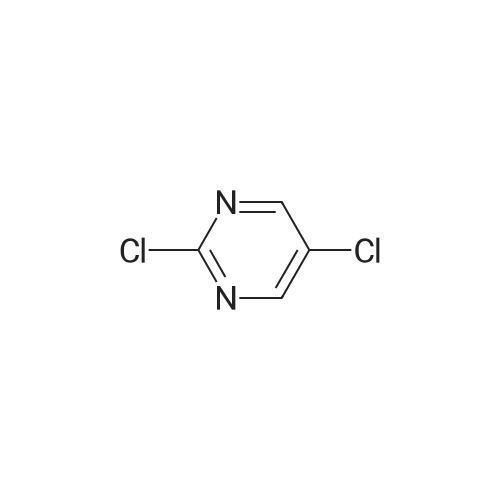
Step 2: Synthesis of 2:2,4,5-Trichloropyrimidine (1.57 eq), 1 (1.0 eq), and potassium carbonate (3.14 eq) in DMF were stirred at 60 °C for 5 hours and then cooled to room temperature. The mixture was filtered and the filtrate was concentrated. The residue was purified by column chromatography (ISCO machine) (DCM/MeOH 20: 1 ) to give 2 as a yellow solid (61 percent yield). |
| 61% |
With potassium carbonate; In N,N-dimethyl-formamide; at 60℃; for 5.0h; |
2,4,5-Trichloropyrimidine (1.57 eq), 1 (1.0 eq), and potassium carbonate (3.14 eq) in DMF were stirred at 60 °C for 5 hours and then cooled to room temperature. The mixture was filtered and the filtrate was concentrated. The residue was purified by column chromatography (ISCO machine) (DCM/MeOH 20:1) to give 2 as a yellow solid (61 percent yield). |
| 50% |
With N-ethyl-N,N-diisopropylamine; for 12.0h;Reflux; |
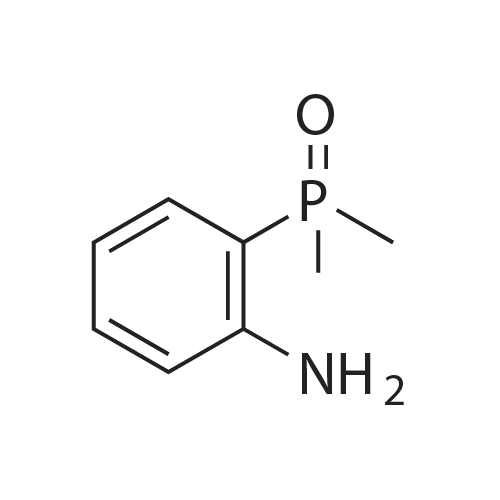
A solution of 2,4,5-trichloropyrimidine(1.82 g, 10 mmol), (2-aminophenyl)dimethylphosphine oxide(1.69 g, 10 mmol) and N,N-diisopropylethylamine (1.94 g, 15 mmol)in propan-2-ol (25 mL) was heated under reflux for 12 h. The solvent was removed by evaporation and the residue was dissolvedin CH2Cl2 (100 mL). The solution was washed with water andsaturated sodium chloride solution and dried, filtered andconcentrated. The residuewas purified by flash chromatography onsilica gel (0e2percent MeOH in DCM) to afford compound 51l (1.60 g,50percent). 1H NMR (400 MHz, DMSO-d6) delta 11.84 (s, 1H), 8.43 (s, 2H), 7.62(s, 2H), 7.25 (s, 1H), 1.83 (s, 3H), 1.80 (s, 3H); 13C NMR (100 MHz,DMSO-d6) d 157.01, 155.99, 142.48, 132.73, 131.47 (d, J 10 Hz),124.17 (d, J 6.0 Hz), 122.64, 122.06 (d, J 3.0 Hz), 121.73, 115.30,18.98, 18.28. HRMS (ESI, m/z) [M+H]+ calcd for C12H13Cl2N3OP:316.0173, found: 316.0175. |
|
With tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate; In N,N-dimethyl-formamide; at 65℃; |
[00369] 245-trichloropyrimidine (54.2 g, 0296 mol, 1.0 eq.), (2arninophenyl)dimethyl..phosphine oxide (50.Og, 0.296 mole, 1.0 eq.), potassium carbonate (49.lg, 0355 mol, 1.2 eq.) and tetrabutylammonium bisuifate (10.2 g. 0.03 mole, 0.1 eq.) were combined in DMF (1050 mL), and heated at 65° C for -8.0-8.5 h. During the course of heating, an offwhite suspension formed. Upon coong, the mixture was cooled to rt and filtered. The coHected solids were rinsed with DMF (2 x 50 mL), and the combined filtrates were concentrated in vacuo. The resulting residue was dissolved in EtOAc (1 .3 L) and water (350 mL). The aqueous layer was isolated and extracted with EtOAc (2 x 250 mL). The combined organic layers were washed with brine (20percent w/w, 500 mL), dried over sodium sulfate, filtered, and concentrated in vacuo to afford (2..((25dichloropyriniidin4 yl)amino)phenyl)dimethylphosphine oxide as an offwhite solid. |
|
With potassium carbonate; In N,N-dimethyl-formamide; at 70℃; |
General procedure: To a solution of 2,4-dichloro-5-fluoropyrimidine (0.5 g, 2.99 mmol) and (2-aminophenyl)dimethylphosphineoxide (0.323 g, 1.907 mmol) in DMF (6.36 mL) wasadded potassium carbonate (0.828 g, 5.99 mmol). The reactionmixture was stirred at 70 °C for overnight. After completion of thereaction, the resulting mixture was cooled to room temperature,quenched with water, extracted with DCM and washed with brine.The combined organic layer was dried over Na2SO4, filtered andconcentrated under reduced pressure. The residue was purified byflash column chromatography on silica gel (0-20percent MeOH in DCM)to give 3f as a yellowish solid (0.178 g, 31percent). |
| 17 g |
at 75℃; for 6.0h; |
2-iodoaniline (17.5 g, 79.9 mmol) and dimethyl phosphine oxide (6.9 g, 88.5 mmol) were added,Palladium acetate (0.3 g, 1.3 mmol), Xantphos (0.77 g, 1.3 mmol),N,N-diisopropylethylamine (22.7 g, 175.8 mmol), DMF (50 mL),Magnetic stirring. Under nitrogen protection, heat to 100°C for 6 hours,The 2-iodoaniline consumption was monitored by thin layer chromatography. Cool to room temperature2,4,5-trichloropyrimidine (17.5 g, 95.9 mmol) was added and the reaction was heated to 75°C for 6 hours.The reaction was complete by thin layer chromatography. Cool to room temperature, add water 300mL,Adjust pH to 5 with 5percent hydrochloric acid and extract with ethyl acetate (100 mL x 3).Wash with sodium bicarbonate solution (100 mL), wash with saturated sodium chloride solution (100 mL × 2),Dry over anhydrous sodium sulfate. It is filtered with suction and concentrated to give a crude brown solid.Recrystallization with ethyl acetate/petroleum ether (volume ratio 1:2) gave an almost white solid 17g.Yield 67.3percent. |
| 0.7 g |
With potassium carbonate; In N,N-dimethyl-formamide; at 60℃; |
Compound b1-1 (0.5 g, 3 mmol) was placed in a 100 mL three-necked flask, and DMF (30 mL) was added.Then, compound b2 (0.86 g, 4.7 mmol), anhydrous potassium carbonate (1.23 g, 9.5 mmol) was added, and stirred at 60 ° C overnight.TLC showed the reaction was almost complete, cooled to room temperature, and then water (100 mL)The organic phase was washed with brine and dried over anhydrous magnesium sulfate.Concentrated through the column to give 0.7 g of a yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping