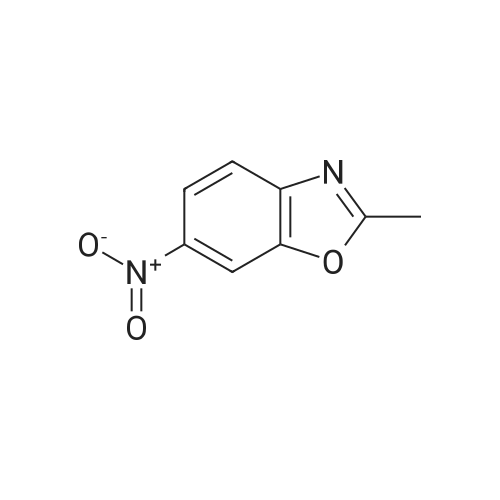
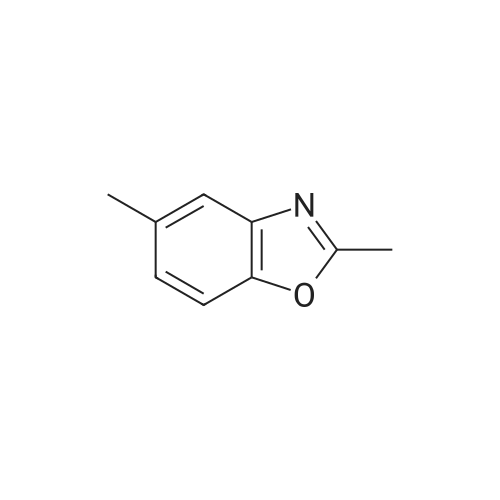
| 85% |
With iron; ammonium chloride; In methanol; water; at 70℃; for 2.0h; |
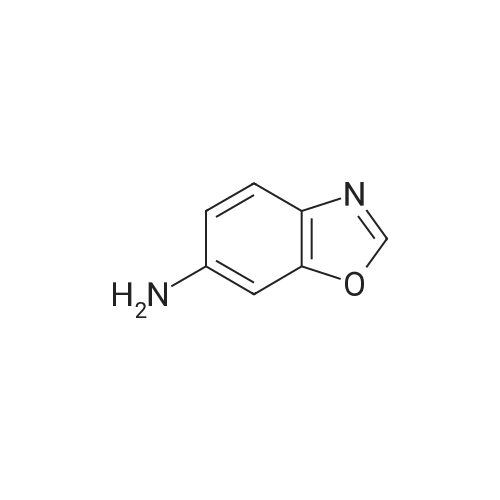
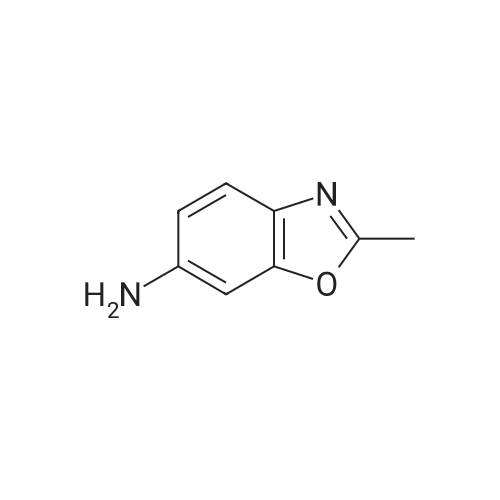
INTERMEDIATE Bl2-Methyl- 1 ,3-benzoxazol-6-amine To a heated suspension (70 0C) of <strong>[5683-43-2]6-nitro-2-methyl-benzoxazole</strong> (4.00 g, 22.4 mmol) in MeOH (60 mL) was added a solution of ammonium chloride (12.1 g, 0.227 mol) in water (40 mL) followed by iron powder (4.52 g, 80.1 mmol). The mixture was stirred for 2 h at 70 0C and then filtered through Celite and washed with MeOH. The filtrate was concentrated and the residue was partitioned between water and EtOAc. The organic layers were combined and the solvent was removed under reduced pressure to give the title compound. Yield 2.83 g (85%). Analytical HPLC: purity 100% (System A); LRESIMS (ESI+) m/z = 149 (M+H)+. |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; for 16.0h; |
2-methylbenzo[d]oxazol-6-amine 2-Methyl-6-nitrobenzoxazole (10.0 g, 56 mmol) and 10% Pd/C (3.4 g) in MeOH (10 mL) were hydrogenated at rt for 16 h. The reaction mixture was filtered through Celite and wash with MeOH (100 mL). The filtrate was concentrated under vacuum to obtained crude 2-methylbenzo[d]oxazol-6-amine (8.4 g), (MS: ESI +ve, 149.07 [M+H]); 1H NMR: (400 MHz, DMSO) delta: 2.47 (s, 3H), 5.24 (s, 2H), 6.56-6.53 (dd, J=2, 1H), 6.70-6.70 (d, J=1.6, 1H), 7.25-7.23 (d, J=8.4, 1H). |
|
With iron; ammonium chloride; In methanol; water; at 70℃; for 4.0h; |
The synthetic route for 1 was outlined in Scheme 1, and the detailed procedures were presented as follows: (a) To a solution of 2-amino-5-nitrophenol (7.70g, 50.0mmol) and pyridine (3.96g, 50.0mmol) in dry xylene (150mL) at 0C was added dropwise acetyl chloride (4.32g, 55.0mmol). The solution was stirred at ambient temperature for 2h. To above solution was added p-toluenesulfonic acid (1.72g, 10.0mmol), the solution was refluxed till no water was discharged. After cooling to room temperature, the solution was washed with water (100mL×3) and a saturated solution of NaCl (50.0mL), respectively. The organic solution was collected and dried over Na2SO4, after evaporation of the solvent, the crude <strong>[5683-43-2]2-methyl-6-nitrobenzoxazole</strong> (pale solid, 8.80g, 95% yield) was obtained for next step without purification. (b) To a solution of 13 <strong>[5683-43-2]2-methyl-6-nitrobenzoxazole</strong> (3.92g, 22.0mmol) in 14 methanol (60.0mL) at 70C was added 15 NH4Cl (11.77g, 220mmol) in 16 H2O (40.0mL) and Fe (4.48g, 80.0mmol). The mixture solution was stirred at 70C for 4h till the starting material disappeared (TLC detection). The mixture solution was cooled to ambient temperature and filtered, the solution was extracted with ethyl acetate (30.0mL×3). The combined organic solution was dried over Na2SO4, after evaporation of the solvent, the crude 17 2-methylbenzoxazol-6-amine (2.77g, 85% yield) was obtained for next step reaction without purification. (c) To a solution of 2-methylbenzoxazol-6-amine (2.66g, 18.0mmol) in methanol (30.0mL) was added 18 formaldehyde (37%, 12mL, 144mmol) and 19 NaBH3CN (2.27g, 36.0mmol). The solution was stirred at ambient temperature for 36h till the starting material disappeared (TLC detection). The solution was poured into H2O (30.0mL), and the mixture solution was extracted with ethyl acetate (30.0mL×3). The combined organic solution was dried over Na2SO4, the solution is concentrated and 20 2-methyl-6-(N, N-dimethylamino) benzoxazole (2.50g, 79% yield) is obtained by flash column chromatography (elute: petroleum ether / ethyl acetate=10 / 1, v/v, Rf =0.11). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping