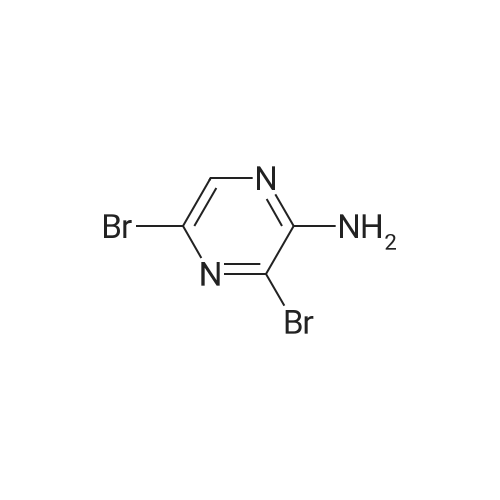
| 70% |
With N-Bromosuccinimide; In acetonitrile; at 0 - 20℃; |
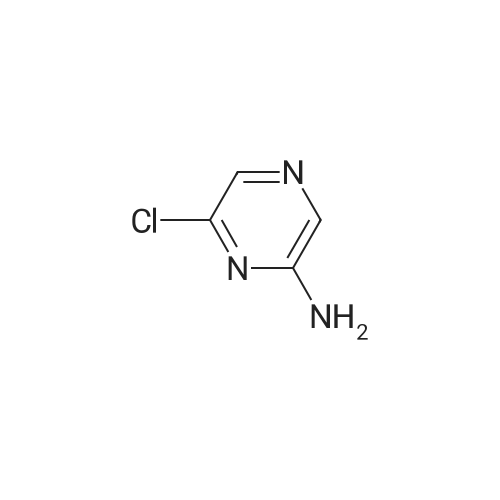
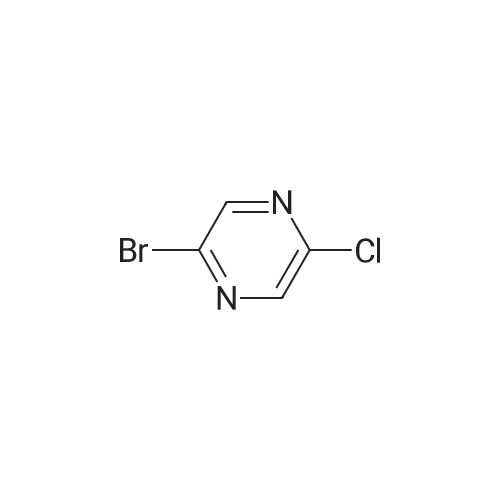
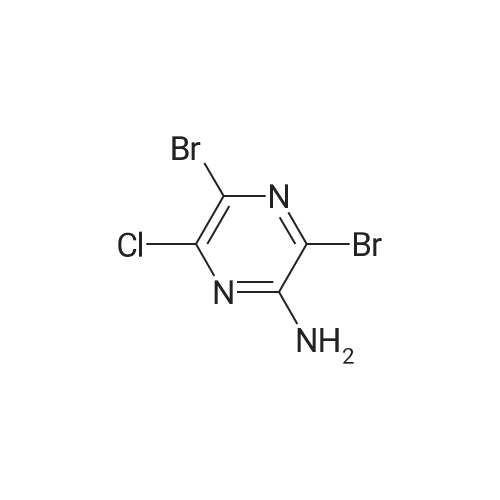
To a solution of <strong>[33332-28-4]2-amino-6-chloropyrazine</strong> (1 g, 7.72 mmol) in anhydrous acetonitrile (10 mL) was gradually added NBS (4.12 g, 23.16 mmol, 3 equiv.) at 0°C. The reaction mixture was slowly warmed up to r.t. and stirred overnight then diluted with water and extracted with Et20. Combined organic layers were washed with water and brine, then dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude material was purified byflash chromatography on silica eluting with Hexane/EtOAc (1:1)to give the title compound (1.55 g, 5.39 mmol, 70percent) as a pale yellow solid. ESI-MS: 287.90 [M+H]+. |
| 38% |
With N-Bromosuccinimide; In chloroform; at 20℃; for 5h;Inert atmosphere; |
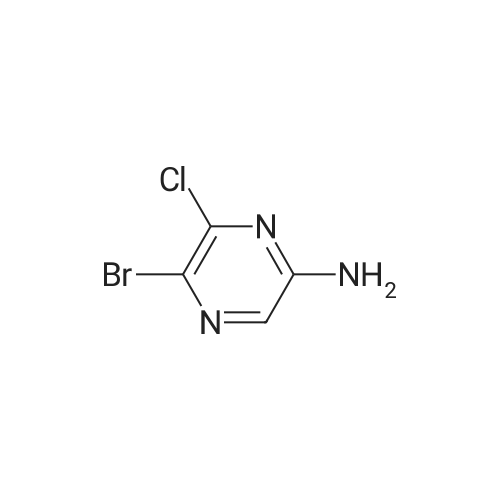
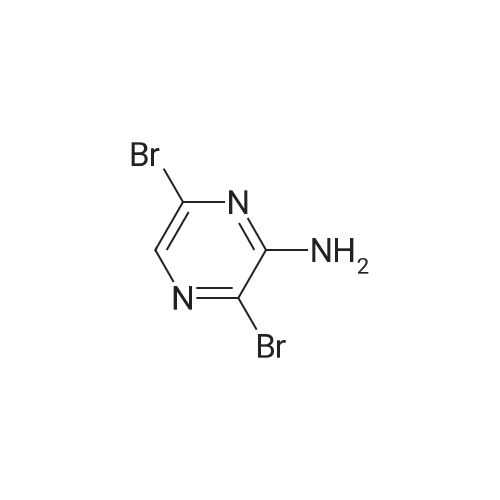
To a solution of 6-chloro-pyrazin-2-ylamine (5.0 g, 38.8 mmol) in chloroform (194 mL) was added N-bromosuccinimide (20.7 g, 116 mmol) under a nitrogen atmosphere and the reaction mixture was stirred at room temperature for 5 h. The resulting mixture was poured into an aqueous solution of K2CO3 (300 mL) and extracted with dichloromethane (200 mL x 4). The combined organic extracts were dried and purified by chromatography (silica, 10-20 percent ethyl acetate in hexanes) to give 3,5-dibromo-6-chloro-pyrazin-2-ylamine (4.2 g, 38percent) as a yellow solid. LC-MS: 286.0 (M-H). |
|
With N-Bromosuccinimide; In chloroform; for 20h;Reflux; |
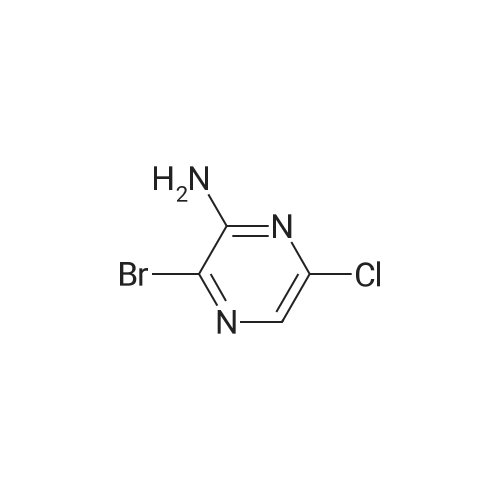
A solution of 6-chloropyrazin-2-amine (2 g, 15.44 mmol) and N BS (13.7 g, 77 mmol) in CHCI3 (100 ml) was heated at reflux for 20 hours. The resulting mixture was purified bychromatography on silica eluting with DCM. The relevant fractions were concentrated in vacuo and the crude product was dissolved in EtOAc (~100 ml), washed with 10 percent sodium thiosulfate (2 x 100 ml), brine, dried (MgS04) and were concentrated in vacuo to afford the title compound; 1 H NMR (400 MHz, CDCI3) delta 5.4-5.0 (2H, br s). |
|
With N-Bromosuccinimide; In methanol; at 15 - 20℃; for 2h;Cooling with ice; |
6-Chloropyrazin-2-amine (100 g, 772 mmol) in MeOH (2000 ml) cooled using a water bath was treated with N-bromosuccinimide (151.2 g, 170 mmol) portionwise over 30 mins, maintaining the reaction temperature between 15-20° C. After stirring for 1.5 hours, the mixture was poured carefully into a stirred vessel of ice-cooled water (4 litres). The resultant suspension was stirred for 2 hours in the ice bath, collected by filtration, rinsing the filter cake with water (800 ml) and dried in a vacuum oven to afford the titled compound; LC-MS Rt 0.99 mins; Method 2 minLowpH |
|
With N-Bromosuccinimide; In chloroform; for 20h;Heating / reflux; |
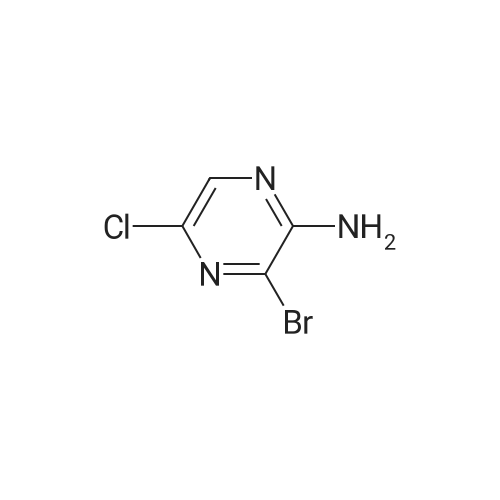
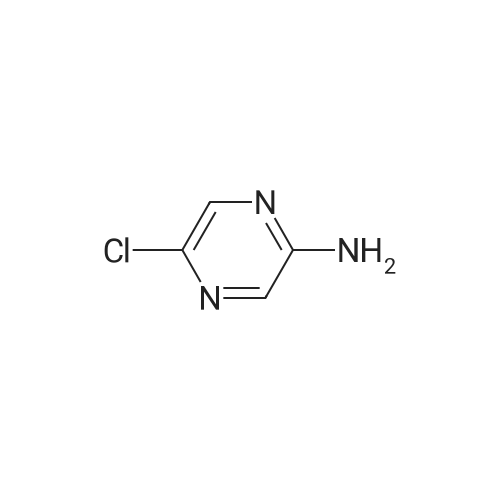
A stirred solution of <strong>[33332-28-4]2-amino-6-chloropyrazine</strong> (2.0g) and JV-bromosuccinimide (13.71g) inchloroform (100 mL) was heated to reflux for 20 hours. The reaction mixture was cooledand concentrated onto silica gel (20g) and the residue loaded onto a column of silica gel(5cm x 2cm) and the column was eluted with dichloromethane. Concentration afforded3,5-dibromo-<strong>[33332-28-4]6-chloro-2-aminopyrazine</strong> that was dissolved into methanol (200 mL) andsodium methoxide (32g of a 25percent solution in methanol) added. The reaction was heated to70°C for 1.5h, cooled and concentrated to approx. 50 mL capacity. The reaction mixturewas poured into water (200mL) and the sub-titled adduct (2.0g) collected as an off-whitesolid.m/e 235, 237 (M+l+, 100percent) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping