| 81.5% |
|
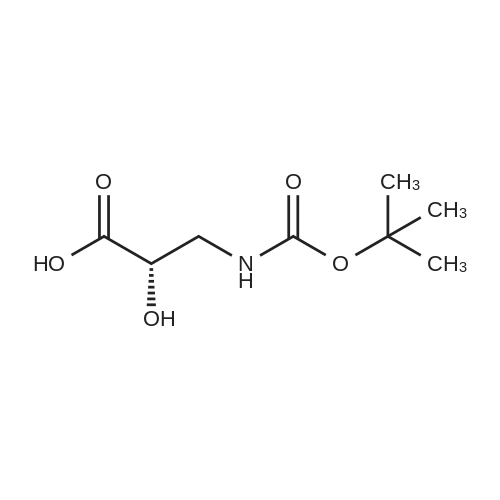
To a stirring solution of S-isoserine (4.0 g, 0.038 mol) in dioxane: 3/40 (100 mL, 1:1 v/v) at 0° C was added N-methylmorpholine (4.77 mL, 0.043 mol), followed by B0C2O (11.28 mL, 0.049 mol) and the reaction was stirred overnight with gradual warming to room temperature. Glycine (1.0 g, 0.013 mol) was then added and the reaction was stirred for 20 min. The reaction was cooled to 0°C and sat aq. NaHC<3/4 (75 mL) was added. The aqueous layer was washed with ethyl acetate (2 x 60 mL) and then acidified to pH 1 with NaHS04. This solution was then extracted with ethyl acetate (3 x 70 mL) and these combined organic layers were dried over Na2SC filtered and concentrated to dryness to give the desired N-Boc-3-amino-2(5)-hydroxy- propanoic acid (6.30 g, 0.031 mmol, 81.5 percent yield): [H NM (400 MHz, CDC13) delta 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m, 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H); 13C NMR (100 MHz, CDC13) delta 174.72, 158.17, 82, 71.85, 44.28, 28.45. |
| 81.5% |
With 4-methyl-morpholine; In 1,4-dioxane; water; at 0 - 20℃; |
N-Boc-3-amino-2(S)-hydroxy-propionic acid ; <n="62"/>To a stirring solution of S-isoserine (4.0 g, 0.038 mol) in dioxane: H2O (100 mL, 1 :1 v/v) at 0° C was added N-methylmorpholine (4.77 mL, 0.043 mol), followed by BoC2O (11.28 mL, 0.049 mol) and the reaction was stirred overnight with gradual warming to room temperature. Glycine (1.0 g, 0.013 mol) was then added and the reaction was stirred for 20 min. The reaction was cooled to 0°C and sat aq. NaHCO3 (75 mL) was added. The aqueous layer was washed with ethyl acetate (2 x 60 mL) and then acidified to pH 1 with NaHSO4. This solution was then extracted with ethyl acetate (3 x 70 mL) and these combined organic layers were dried over Na2SO4, filtered and concentrated to dryness to give the desired N-Boc-3-amino-2(5)-hydroxy- propanoic acid (6.30 g, 0.031 mmol, 81.5 percent yield): 1H NMR (400 MHz, CDC13) delta 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m, 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H); 13C NMR (100 MHz, CDC13) delta 174.72, 158.17, 82, 71.85, 44.28, 28.45. |
| 81.5% |
|
N-Boc-3-amino-2 (^-hydroxy-propionic acid OHTo a stirring solution of S-isoserine (4.0 g, 0.038 mol) in dioxane: H2O (100 mL, 1 :1 v/v) at 0° C was added N-methylmorpholine (4.77 mL, 0.043 mol), followed by BoC2O (11.28 mL, 0.049 mol) and the reaction was stirred overnight with gradual warming to room temperature. Glycine (1.0 g, 0.013 mol) was then added and the reaction was stirred for 20 min. The reaction was cooled to 0°C and sat aq. NaHCO3 (75 mL) was added. The aqueous layer was washed with ethyl acetate (2 x 60 mL) and then acidified to pH 1 with NaHSO4. This solution was then extracted with ethyl acetate (3 x 70 mL) and these combined organic layers were dried over Na2SO4, filtered and concentrated to dryness to give the desired N-Boc-3-amino-2(5)-hydroxy- propanoic acid (6.30 g, 0.031 mmol, 81.5 percent yield): 1H NMR (400 MHz, CDC13) delta 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m, 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H); 13C NMR (100 MHz, CDC13) delta 174.72, 158.17, 82, 71.85, 44.28, 28.45. |
| 81.5% |
With 4-methyl-morpholine; In 1,4-dioxane; water; at 0 - 20℃; |
N-Boc-3-amino-2(5)-hydroxy-propionic acid To a stirring solution of S-isoserine (4.0 g, 0.038 mol) in dioxane: H2O (100 mL, 1:1 v/v) at 0° C was added N-methylmorpholine (4.77 mL, 0.043 mol), followed by BoC2O (11.28 mL, 0.049 mol) and the reaction was stirred overnight with gradual warming to room temperature. Glycine (1.0 g, 0.013 mol) was then added and the reaction was stirred for 20 min. The reaction was cooled to 0°C and sat aq. NaHCO3 (75 mL) was added. The aqueous layer was washed with ethyl acetate (2 x 60 mL) and then acidified to pH 1 with NaHSO4. This solution was then extracted with ethyl acetate (3 x 70 mL) and these combined organic layers were dried over Na2SO4, filtered and concentrated to dryness to give the desired N-Boc-3-amino-2(5)-hydroxy- propanoic acid (6.30 g, 0.031 mmol, 81.5 percent yield): 1H NMR (400 MHz, CDC13) delta 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m, 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H); 13C NMR (100 MHz, CDC13) delta 174.72, 158.17, 82, 71.85, 44.28, 28.45. |
| 80% |
|
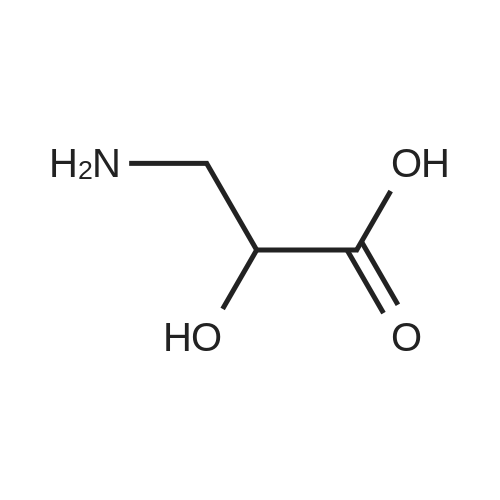
Part I - Synthesis of (S)-3-((ter^butoxycarbonyl)amino)-2-hydroxypropanoic acid [0261] To (L)-isoserine (1.0 g, 9.5 mmol) in tetrahydrofuran (10 mL) and 2M sodium hydroxide (9.75 mL, 19.5 mmol) was added di-tert-butyl dicarbonate (0.78 g, 10 mmol). The resulting mixture was stirred vigorously at ambient temperature overnight. Then, the reaction mixture was acidified with 1M hydrogen chloride (20 mL) and stirred for 20 minutes until gas evolution ceased. The resulting mixture was partitioned between ethyl acetate and water, washed with brine, dried with sodium sulfate, filtered and concentrated in vacuo to yield title compound. (1.57 g, 80percent yield) |
|
|
To a stirring solution of S-isoserine (4.0 g, 0.038 mol) in dioxane: H20 (100 mL, 1 : 1 v/v) at 0° C was added N-methylmorpholine (4.77 mL, 0.043 mol), followed by Boc20 (1 1.28 mL, 0.049 mol) and the reaction was stirred overnight with gradual warming to room temperature. Glycine (1.0 g, 0.013 mol) was then added and the reaction was stirred for 20 min. The reaction was cooled to 0°C and sat aq. NaHC03 (75 mL) was added. The aqueous layer was washed with ethyl acetate (2 x 60 mL) and then acidified to pH 1 with NaHS04. This solution was then extracted with ethyl acetate (3 x 70 mL) and these combined organic layers were dried over Na2S04, filtered and concentrated to dryness to give the desired N-Boc-3-amino-2(5)-hydroxy- propanoic acid (6.30 g, 0.031 mmol, 81.5 percent yield): 1H NMR (400 MHz, CDC13) delta 7.45 (bs, 1 H), 5.28 (bs, 1 H), 4.26 (m, 1 H), 3.40-3.62 (m, 2 H), 2.09 (s, 1 H), 1.42 (s, 9 H); 13C NMR (100 MHz, CDC13) delta 174.72, 158.17, 82, 71.85, 44.28, 28.45. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping