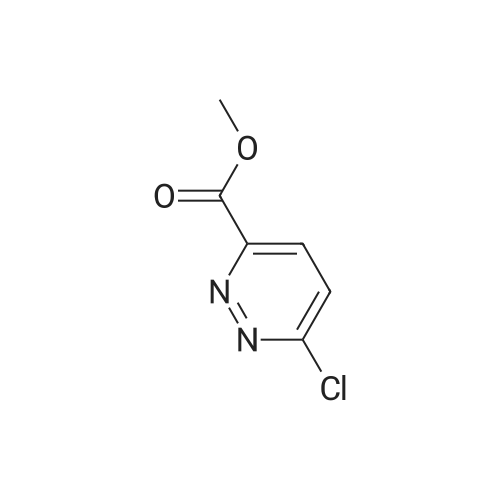
| 29.0 g |
In methanol;Inert atmosphere; Reflux; |
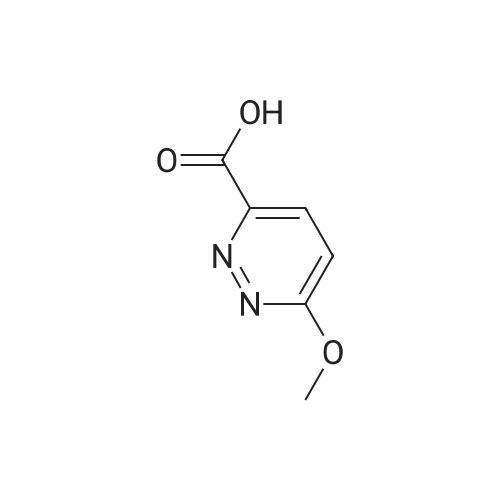
In a nitrogen atmosphere, a mixture of <strong>[65202-50-8]methyl 6-chloropyridazine-3-carboxylate</strong> (40 g), sodium methoxide (62.5g) and methanol (600 mL) was heated to reflux overnight. The reaction mixture was cooled to room temperature.Hydrochloric acid was added to the reaction mixture at 0°C to adjust the pH to 3. The solvent was distilled off, and then,the residue was dissolved in water (200 mL), followed by extraction with ethyl acetate (300 mL) three times. The combinedorganic layer was washed with saturated brine, then dried over sodium sulfate and concentrated. The residue waswashed with methyl t-butyl ether/petroleum ether (1:5) to obtain 6-methoxypyridazine-3-carboxylic acid (29.0 g). A mixtureof 6-methoxypyridazine-3-carboxylic acid (29.0 g), sulfuric acid (2 mL) and methanol (500 mL) was stirred at roomtemperature for 3 days. A saturated aqueous solution of sodium bicarbonate was added to the reaction mixture to adjustthe pH to 7. The solvent was distilled off, and then, the residue was dissolved in water (200 mL), followed by extractionwith ethyl acetate (200 mL) three times. The combined organic layer was washed with saturated brine, then dried oversodium sulfate and concentrated. The obtained residue was purified by column chromatography (ethyl acetate/petroleumether) to obtain methyl 6-methoxypyridazine-3-carboxylate (22.0 g). A mixture of methyl 6-methoxypyridazine-3-carboxylate(4.5 g), hydrazine hydrate (80percent, 13.4 g) and methanol (50 mL) was stirred overnight at room temperature. Water(10 mL) was added to the reaction mixture, and the mixture was concentrated. Methanol was distilled off from the mixture,and the residue was freeze-dried. The residue was washed with petroleum ether to obtain 6-methoxypyridazine-3-carbohydrazide (4.2 g). 2-Chloro-2-oxoethyl acetate (16.3 g) was added dropwise to a mixture of 6-methoxypyridazine-3-carbohydrazide (20 g), dichloromethane (250 mL) and water (10 mL) at 0°C. The mixture was stirred at 0°C for 30minutes and then stirred at room temperature for 1.5 hours. The reaction mixture was concentrated under reducedpressure. Water (20 mL) was added to the obtained residue, and the mixture was freeze-dried. The residue was washedwith petroleum ether to obtain 2-(2-((6-methoxypyridazin-3-yl)carbonyl)hydrazino)-2-oxoethyl acetate (25.0 g). A mixtureof 2-(2-((6-methoxypyridazin-3-yl)carbonyl)hydrazino)-2-oxoethyl acetate (25.0 g), diphosphorus pentasulfide (21.4 g)and THF (600 mL) was stirred overnight at 50°C. The reaction mixture was cooled to room temperature, and the reactionwas terminated by the addition of a saturated aqueous solution of sodium bicarbonate. The mixture was subjected toextraction with ethyl acetate (500 mL) three times. The combined organic layer was washed with saturated brine, thendried over sodium sulfate and concentrated. The obtained residue was purified by column chromatography (dichloromethane/methanol) to obtain (5-(6-hydroxypyridazin-3-yl)-1,3,4-thiadiazol-2-yl)methyl acetate (7.9 g). Amixture of(5-(6-hydroxypyridazin-3-yl)-1,3,4-thiadiazol-2-yl)methyl acetate (7.9 g), phosphoryl chloride (9.6 g) and acetonitrile (200 mL) was stirred at 70°C for 2 hours. The reaction mixture was cooled to room temperature. The reaction was terminatedby the addition of water under ice cooling, and the mixture was subjected to extraction with ethyl acetate (200 mL) threetimes. The combined organic layer was washed with saturated brine, then dried over sodium sulfate and concentrated.The obtained residue was purified by column chromatography (ethyl acetate/petroleum ether) to obtain (5-(6-chloropyridazin-3-yl)-1,3,4-thiadiazol-2-yl)methyl acetate (2.9 g). To a mixture of (5-(6-chloropyridazin-3-yl)-1,3,4-thiadiazol-2-yl)methyl acetate (1.6 g) and THF (50 mL), lithium hydroxide (1 M aqueous solution, 14.8 mL) was added at 0°C. Themixture was stirred at 0°C for 1 hour and then stirred at room temperature for 1 hour. The solvent was distilled off, andthen, the residue was subjected to extraction with ethyl acetate (50 mL) three times. The combined organic layer waswashed with saturated brine, then dried over sodium sulfate and concentrated. The obtained residue was recrystallizedfrom dichloromethane-methanol to obtain (5-(6-chloropyridazin-3-yl)-1,3,4-thiadiazol-2-yl)methanol (1.16 g). A mixtureof 4-(4-(pentafluorosulfanyl)phenyl)-4-(trifluoromethyl)piperidine hydrochloride (200 mg), (5-(6-chloropyridazin-3-yl)-1,3,4-thiadiazol-2-yl)methanol (100 mg), DIPEA (0.650 mL) and IPA (4 mL) was stirred at 150°C for 1 hour underirradiation with microwave. The reaction mixture was concentrated under reduced pressure. The obtained residue waspurified by column chromatography (ethyl acetate/hexane) to obtain the title compound (120 mg). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping