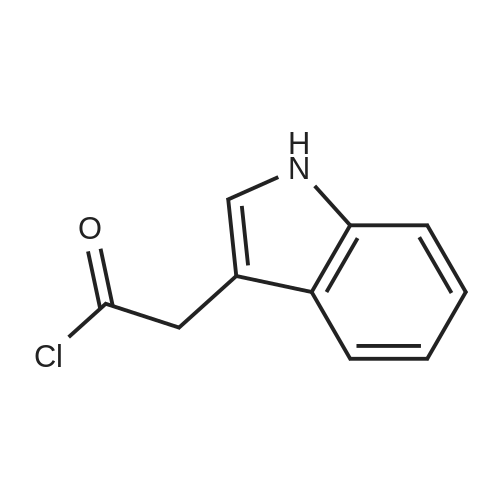
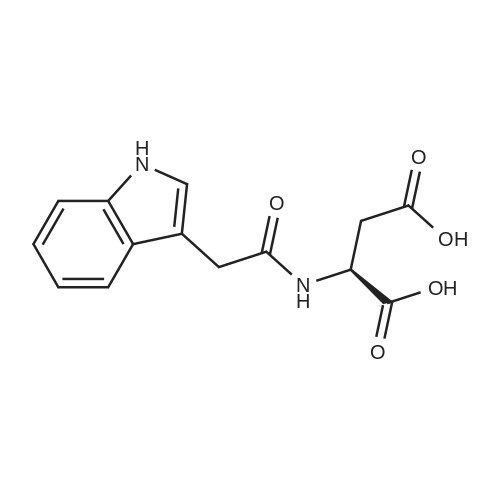
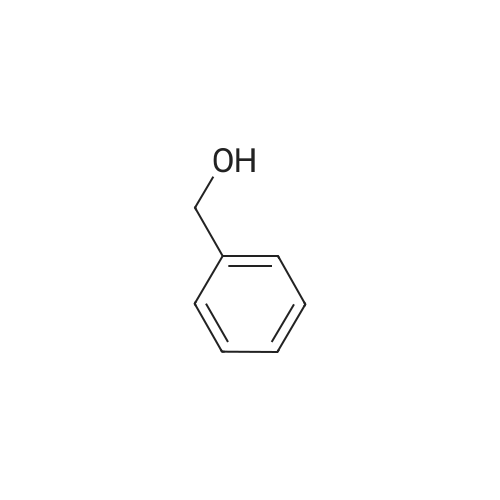
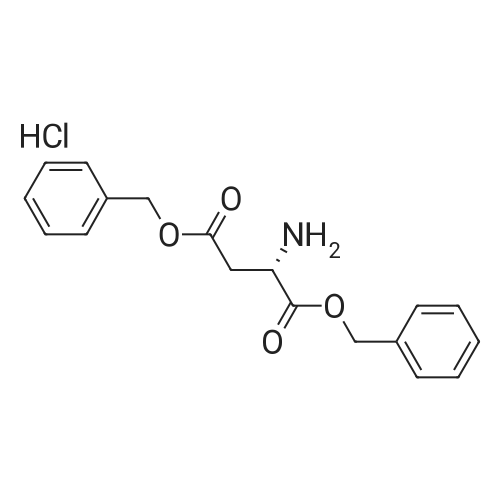
| 100% |
|
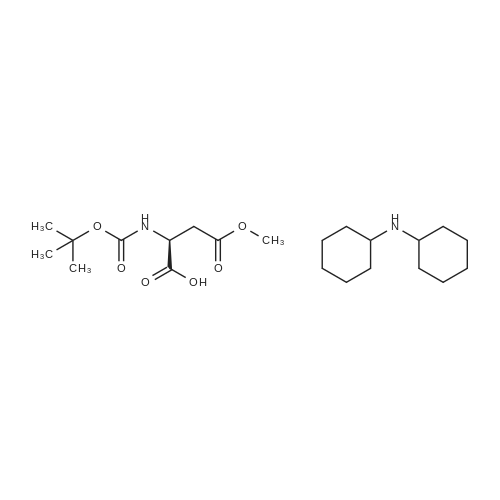
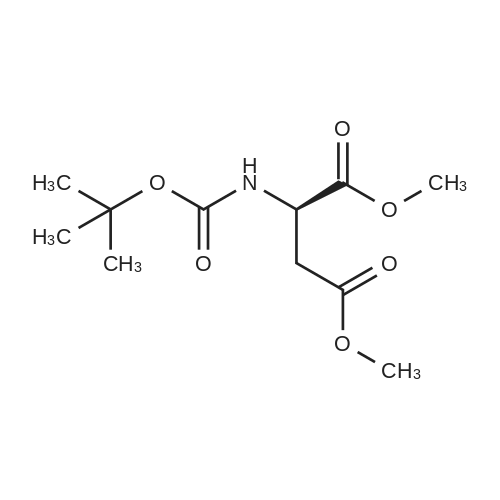
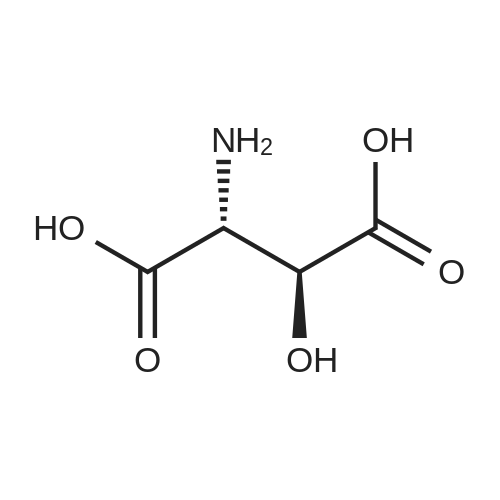
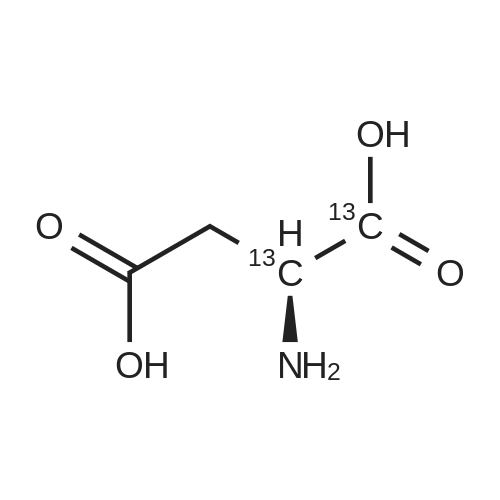
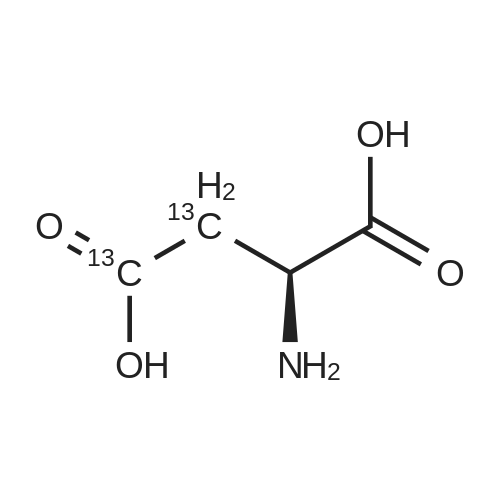
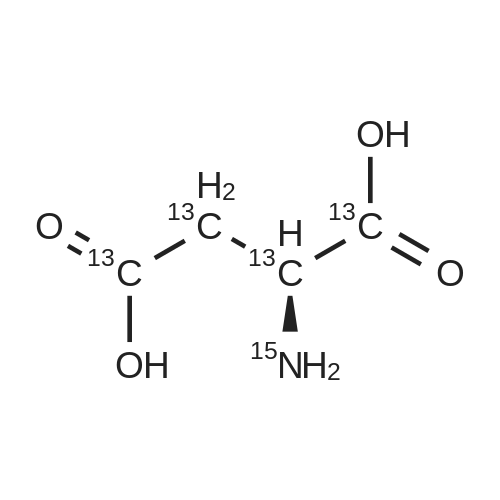
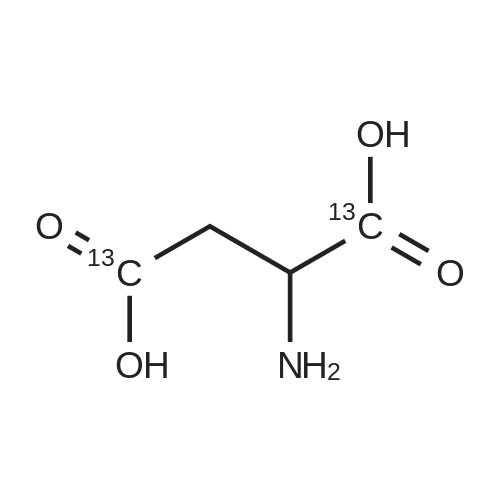
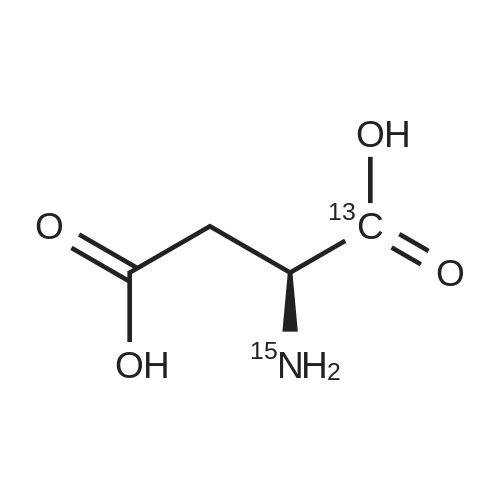
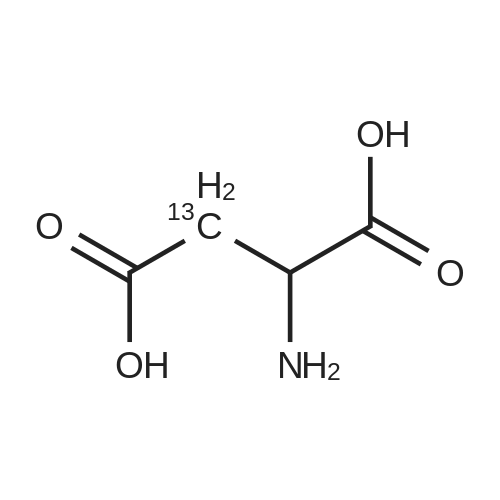
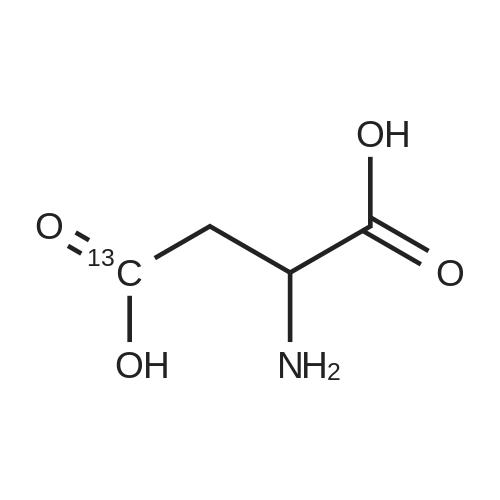
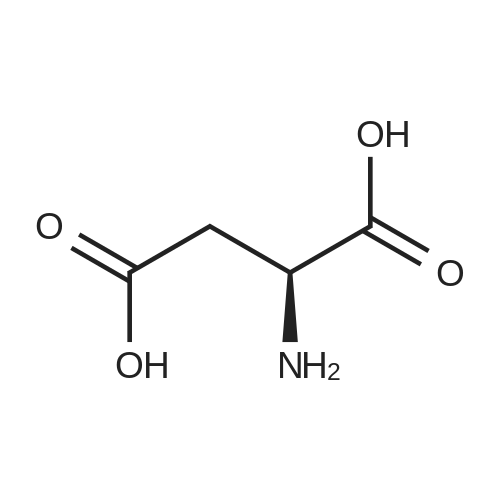
Chlorotrimethylsilane (21.2 mL, 165.4 mmol) was added slowly to a stirred suspension of l-aspartic acid (5) (5.00 g, 37.6 mmol) in methanol (100 mL) at 0 C. The reaction mixture was allowed to stir for 1 h at 0 C and then at room temperature for 24 h. Triethylamine (34.0 mL, 244.4 mmol) and di-tert-butyl dicarbonate (9.02 g, 41.4 mmol) were added slowly and the mixture stirred for 2 h. The reaction mixture was concentrated in vacuo and the resulting residue dissolved in diethyl ether (200 mL) and filtered to remove the white precipitate. The filtrate was concentrated in vacuo and purified using flash column chromatography (35% ethyl acetate in petroleum ether) to yield dimethyl (2S)-2-(tert-butoxycarbonylamino)butane-1,4-dioate (6) as a white solid (9.81 g, 100%). Mp 58-60 C; numax (NaCl) 3406 (NH), 2927 (CH), 2360, 1704 (C=O), 1458, 1159, 1045 cm-1; [alpha]D25 +35.8 (c 1.0, CHCl3), lit. 9a [alpha]D25 +30.8 (c 2.1, CHCl3); deltaH (400 MHz, CDCl3) 1.47 (9H, s, tBu), 2.85 (1H, dd, J 16.8, 4.2 Hz, 3-HH), 3.03 (1H, dd, J 16.8, 4.2 Hz, 3-HH), 3.72 (3H, s, OMe), 3.78 (3H, s, OMe), 4.58-4.61 (1H, m, 2-H), 5.50 (1H, br d, J 8.4 Hz, NH); deltaC (101 MHz, CDCl3) 28.3 (3*CH3), 36.7 (CH2), 49.9 (CH), 52.1 (CH3), 52.8 (CH3), 80.2 (C), 155.4 (C), 171.5 (C), 171.6 (C); m/z (CI) 262 (MH+, 15%), 206 (100), 162 (36), 85 (13); HRMS (CI): MH+, found 262.1293. C11H20NO6 requires 262.1291. |
| 92% |
|
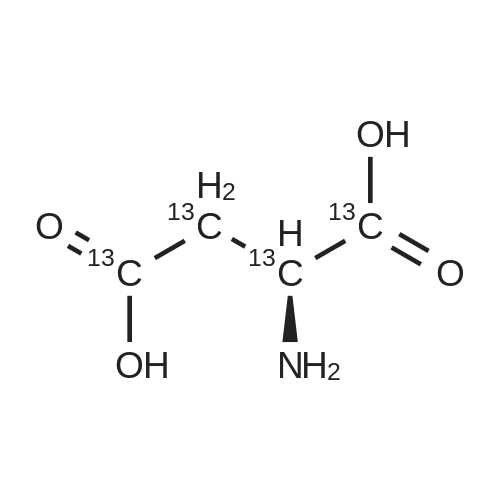
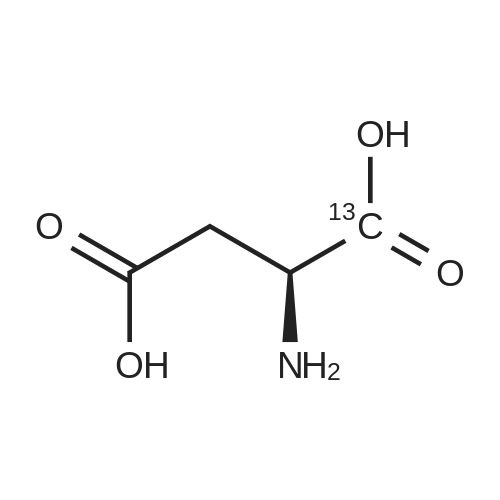
L-Aspartic acid (1 ; 5.002 g, 37.58 mmol) was added to an oven-dried round-bottom flask and placed under an atmosphere of dry N2(g). The starting material was then dissolved partially with 60 mL of anhydrous methanol, and the mixture was cooled to 0 C. Once the desired temperature was reached, thionyl chloride (8.2 mL, 1 10 mmol) was added drop-wise. After the addition was complete, the reaction mixture became homogenous, and was warmed slowly to room temperature and left to stir for 14 h. The reaction mixture was then concentrated under reduced pressure, and the resulting diester was dissolved in 150 mL of DCM and 100 mL of water. To this biphasic solution was added sodium bicarbonate (4.212 g, 50.14 mmol) and di-f-butyl dicarbonate (9.841 g, 45.09 mmol), and the reaction mixture was heated at reflux for 4 h. After the reaction was confirmed to be complete by TLC, the reaction mixture was allowed to cool to room temperature. The organic layer was separated, and the aqueous layer was extracted three times with 150 mL of DCM. The organic extracts were combined, washed with 250 mL of saturated NaCI(aq), dried over MgS0 (s), and concentrated under reduced pressure. Flash chromatography (35% v/v ethyl acetate in hexanes) was used to isolate 2 [(S)-dimethyl 2-(teri-butoxycarbonylamino)succinate] as a white solid (9.080 g, 92%, 2 steps). [00159] 1H NMR (400 MHz, CDC13) delta = 5.49 (d, J = 8.3 Hz, 1H), 4.60-4.57 (m, 1H), 3.76 (s, 3H), 3.70 (s, 3H), 3.01 (dd, J = 17, 4.4 Hz, 1H), 2.83 (dd, J = 17.0, 4.7), 1.45 (s, 9H); 13C NMR (75 MHz, CDC13) delta = 171.6, 171.5, 155.5, 80.3, 52.8, 52.1, 50.0, 36.8, 28.4; HRMS (ESI) calculated for [CnHi9N06Na]+ (M+Na+) requires mlz = 284.1105, found 284.1113 |
|
|
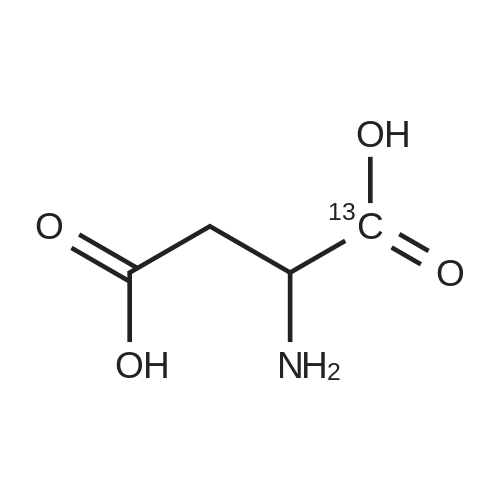
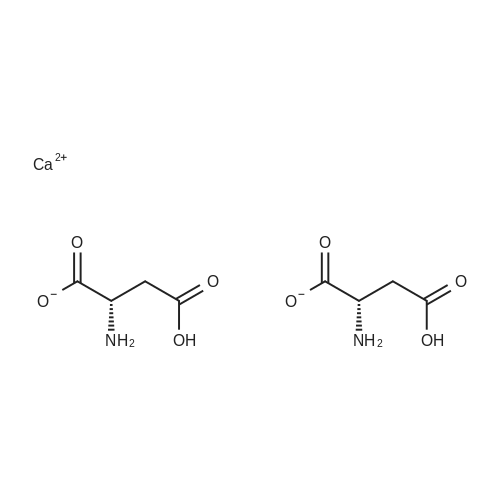
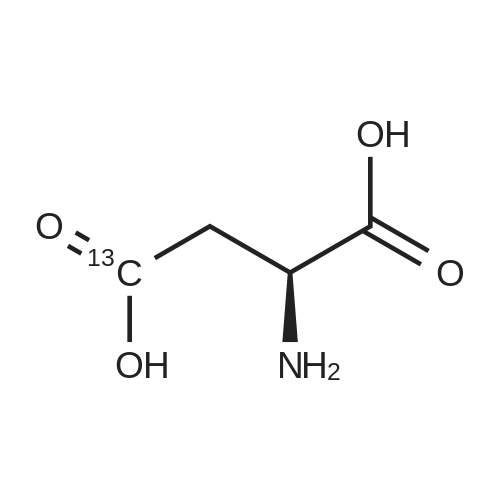
A 0 C suspension of L-aspartic acid (10.1 g, 76.0 mmol) in anhydrous methanol (100 mL) was treated with trimethylsilyl chloride (24.7 g, 228 mmol) via rapid dropwise addition. The cooling bath was removed and the resulting solution stirred at ambient temperature overnight (16 h) and then concentrated under reduced pressure. The resulting oil was taken up in dichloromethane (100 mL), treated with di-tert-butyl dicarbonate (17.4 g, 80.1 mmol) and diisopropylethylamine (26.5 mL, 152 mmol) and stirred at ambient temperature overnight (16 h). The resulting solution was washed with 0.25 N aqueous hydrochloric acid (3x50 mL), 0.25 N aqueous sodium hydroxide (3x50 mL), and brine (1x50 mL); dried over MgSO4 and concentrated under reduced pressure to afford (S)-dimethyl 2-((tert-butoxycarbonyl)amino)succinate (15.9 g, 60.9 mmol, 80% crude yield) as a near colorless oil. The crude product was used in the next step without further purification. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping