| 64% |
|
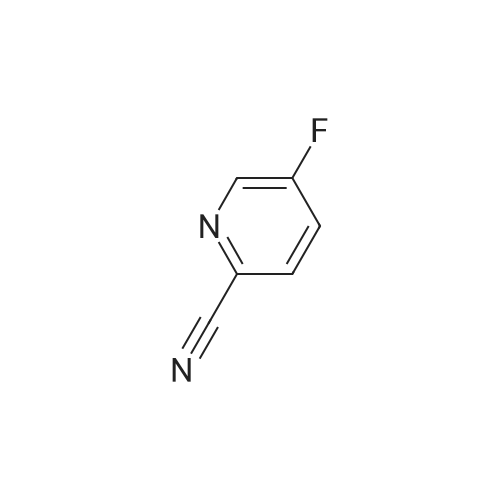
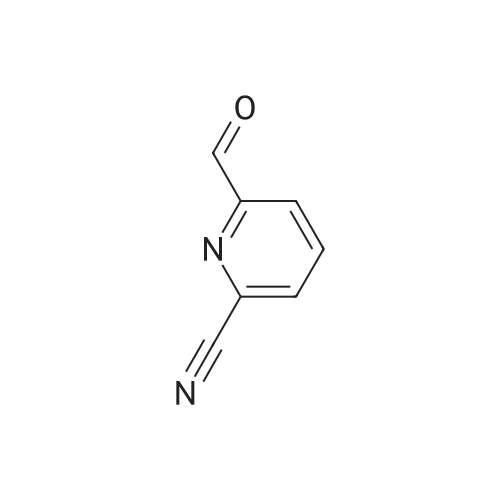
To hydrogen fluoride - pyridine (100 ml) was added 5-amino-2-cyanopyridine (24.5 g, 0.206 mol) under ice cooling. The resulting mixture was stirred for 10 minutes. To the reaction mixture was added sodium nitrite (15.6 g, 0.226 mol). The resulting mixture was stirred at room temperature for 10 minutes, and then stirred at 50C for 2 hours. To the reaction mixture was added a 20% aqueous solution of sodium hydroxide. The resulting mixture was extracted with diethyl ether. The organic layer thus obtained was dried over sodium sulfate, and concentrated under reduced pressure. The residue thus obtained was subjected to silica gel column chromatography. The fraction obtained from the hexane:ethyl acetate=3:1 eluate was concentrated under reduced pressure to give the title compound (16.0 g, 0.131 mmol, 64%) as colorless needle crystals. 1H-NMR(400MHz,CDCl3)δ: 7.57(1H,ddd,J=8.6,8.6,3.1Hz), 7.77(1H,dd,J=8.6,4.4Hz), 8.60(1H,d,J=3.1Hz). IR(ATR)cm-1: 3095, 2237, 1577, 1467, 1409, 1375, 1272, 1240, 1197, 1120, 1010. MSm/z: 122 (M+). EI-MS: 122.0293 (Calcd for C6H3FN2: 122.0280). |
| 64% |
|
1) 5-Fluoropyridine-2-carbonitrile 5-Amino-2-cyanopyridine (24.5 g) was added to hydrogen fluoride-pyridine (100 mL) under ice cooling, and the resultant mixture was stirred for 10 minutes. Sodium nitrite (15.6 g) was added to the reaction solution, and the mixture was stirred at room temperature for 10 minutes, and then stirred at 50C for 2 hours. After air cooling, a 20% aqueous solution of sodium hydroxide and diethyl ether were added to the reaction solution, and the resultant mixture was partitioned. The organic layer was dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and a residue thus obtained was purified by silica gel column chromatography (hexane-ethyl acetate), to obtain 5-fluoropyridine-2-carbonitrile (16.0 g, 64%) as a solid. 1H-NMR (400MHz, CDCl3) δ: 7.57 (1H, ddd, J=8.6, 8.6, 3.1Hz), 7.77 (1H, dd, J=8.6, 4.4Hz), 8.60 (1H, d, J=3.1Hz). MS (EI) m/z: 122 (M+). |
| 52% |
With pyridine hydrogenfluoride; sodium nitrite; at 0 - 65℃;Inert atmosphere; |
To a 100 mL round bottom flask charged with 5-aminopicolinonitrile (285 mg, 2.44 mmol) at 0 C. was added HF-pyridine (5 mL) under N2. A pale brown solution was formed. In 4 aliquots, NaNO2 (250 mg, 3.62 mmol) was added with stirring. The solution turned green and a brown gas was liberated. After 20 min at 0 C., the solution was allowed to reach rt and stirred for an additional 20 min. A reflux condenser was attached and the reaction mixture was heated at 65 C. for 20 min then allowed to cool to rt. The orange slurry was quenched by the addition of crushed ice and the aqueous layer was extracted with DCM (3×10 mL). The combined organic portions were dried over Na2SO4, decanted and concentrated under reduced pressure to yield 5-fluoropicolinonitrile (152 mg, 52% yield) as a pale orange powder. LCMS: RT=0.63 min [M+H] 122.9 (2 min Phenomenex Luna C18 column, 4.6×30 mm eluting with 10-90% MeOH/H2O over 2 minutes containing 0.1% TFA; 5 mL/min, monitoring at 220 nm); HPLC: RT=0.99 min (Phenomenex Luna C18 column, 4.6×50 mm eluting with 10-90% MeOH/H2O over 4 minutes containing 0.2% PPA; 4 mL/min, monitoring at 220 nm, Purity 96%); NMR: 400 MHz 1H (CDCl3) ppm 8.52 (d, J=2.64 Hz, 1H), 7.70 (dd, J=4.4 and J=8.36 Hz, 1H), 7.50 (m, 1H). |
|
With pyridine hydrogenfluoride; sodium nitrite; at -10 - 80℃; for 2.75h; |
EXAMPLE 10; Example 10 utilized a 5-fluoro-2-hydroxyamidinylpyridine as an intermediate to obtain the desired product. To a mixture of 5-amino-2-cyanopyridine (100 g, 840 rnmol) cooled to -1O0C was added HF-pyridine (50OmL, 70%v/v). Sodium nitrite (91g, 1.32mol) was added in portions. The reaction was then stirred at -100C for 45 minutes, room temperature for 30 minutes, and 800C for 90 minutes. Upon completion, the reaction was cooled to room temperature and quenched with ice/water. The aqueous solution was extracted with CH2Cl2, dried over magnesium sulfate and concentrated. The fluoropyridine (4Og, 328mmol) was treated with sodium carbonate (82g, 773mmol) and hydroxylamine- hydrochloride (45g, 652mmol) in methanol (30OmL). The reaction was allowed to stir for 24h and upon completion, the reaction was concentrated in vacuo, diluted with water, filtered and dried under vacuum.Example 10 was generated under similaτ reaction conditions described in the examples above and shown in Schemes 4 and 5. 1H NMR (DMSOd6, 500 MHz) δ 12.0 (s, IH), 8.79(s, IH), 8.23 (m, IH), 8.14 (m, IH), 7.97 (m, IH), 7.64 (m, IH), 7.26 (m, IH), 4.64 (m, IH), 3.56 (m, 2H); LCMS m/z 394 (M+Na). |
|
With pyridine; hydrogen fluoride; sodium nitrite; at 0 - 80℃; for 2.75h; |
EXAMPLE 17As shown in Scheme 6 a suspension of 5-amino-2-cyano pyridine (20.0 g, 0.168 mol) in HF-pyridine (100 g) in an Erlenmeyer flask cooled to O0C was added sodium nitrite (17.4 g, 0.25 mol) in four portions. After 45 min at O0C the reaction mixture was stirred at room temperature for 30 min and then heated to 8O0C for 90 min. The reaction mixture was quenched by pouring into ice/water mixture. The resulting mixture was extracted with DCM. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give the fluoropyridine as an orange solid.To a suspension of this fluoropyridine nitrile intermediate (16.0 g, 0.13 mol) in methanol (200 mL) was added hydroxylamine (9.63 mL, 0.16 mmol, 50% by wt). After stirring the reaction mixture at room temperature for 48 h, it was filtered through a fritted funnel. The precipitate was washed with ether and dried under vacuum to give the N-hydroxy amidine as a yellow solid.To a suspension of this amidine intermediate (5.32 g, 34.3 mmol) in anhydrous pyridine (10 mL) was added 4-chloro-4-oxo-methyl butyrate (5 mL, 41.2 mmol). The resulting reaction mixture was heated at 12O0C for 2 h. The mixture was cooled to RT and concentrated. The residue was dissolved in ethyl acetate and washed with IN HCl, water and brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give a dark brown solid. This material was purified by Biotage using 25%-60% ethyl acetate-hexanes gradient to give the heterobiaryl intermediate as a light yellow solid.To a solution of this ester intermediate (900 mg, 3.58 mmol) in THF (4 mL) was added methanol (2 mL) followed by 5N NaOH (1 mL). After 30 min, the reaction mixture was EPO <DP n="41"/>neutralized by the addition of IN HCl (5 mL). The reaction mixture was concentrated. The residue was extracted with ethyl acetate, and the organic layer was washed with brine, dried over anhydrous sodium sulfate, filtered and concentrated to give a light yellow solid of the carboxylic acid.EXAMPLE 17 was prepared by reaction of the carboxylic acid described above and the intermediate prepared for EXAMPLE 2. 1H NMR (DMSOd6, 500 MHz) δ 10.95 (br s, IH), 8.75 (d, IH), 8.12 (dd, IH), 7.95-7.91 (m, IH) .50 (t, 2H), 3.31 (t, 2H), 3.03 (d, IH), 2.32- 2.21 (m, 3H), 1.75 (br s, 2H), 1.26-1.24 (m, lH)m 0.99 (d, 3H); LCMS m/z 372 (M+l). |
|
With pyridine hydrogenfluoride; sodium nitrite; at 0 - 80℃; for 2.75h; |
To a suspension of 5-amino-2-cyano pyridine (20-0 g, 0.168 mol) in HF-pyridine (100 g) in an Erlenmeyer flask cooled to 0 C. was added sodium nitrite (17.4 g, 0.251 mol) in four portions. After 45 min at 0 C. the reaction mixture was stirred at room temperature for 30 min and then heated to 80 C. for 90 min. The reaction mixture was quenched by pouring into an ice/water mixture. The resulting mixture was extracted with DCM. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give the fluoropyridine nitrile as an orange solid. To a suspension of this fluoropyridine nitrile intermediate (16.0 g, 0.131 mol) in methanol (200 mL) was added hydroxylamine (9.63 mL, 0.157 mmol, 50% by wt). After stirring the reaction mixture at room temperature for 48 h, it was filtered through a fritted funnel. The precipitate was washed with ether and dried under vacuum to give the N-hydroxyamidine as a yellow solid. To a suspension of this amidine intermediate (5.32 g, 34.32 mmol) in anhydrous pyridine (10 mL) was added 4-chloro-4-oxo-methyl butyrate (5 mL, 41.18 mmol). The resulting reaction mixture was heated at 120 C. for 2 h. The mixture was cooled to RT and concentrated. The residue was dissolved in ethyl acetate and washed with 1N HCl, water and brine. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give a dark brown solid. This material was purified by Biotage using 25%-60% ethyl acetate-hexanes gradient to give the heterobiaryl intermediate as a light yellow solid. To a solution of this ester intermediate (900 mg, 3.58 mmol) in dioxane (3 mL) was added ammonium hydroxide (3 mL) and the mixture was allowed to stir at room temperature for 12 hours. Upon completion, the mixture was concentrated, and the amide was purified via flash chromatography (Biotage 40M). To the amide (0.25 g, 1.0 mmol) in a degassed solution of dioxane (7 mL) was added the corresponding triflate (0.92 g, 2.1 mmol), cesium carbonate (1.0 g, 3.0 mmol), xantphos ligand (0.1 g, 0.2 mmol), and Pd2(dba)3 catalyst (0.09 g, 0.1 mmol), and the reaction mixture was heated to 75 C. for 6 hours. The mixture was cooled, filtered, concentrated in vacuo, and purified via flash chromatography (Biotage 40 M). To the desired cycloalkene (0.26 g, 0.5 mmol) in THF/H2O (1:1) was added sodium hydroxide (0.06 g, 1.5 mmol). The biphasic reaction mixture was allowed to stir for 12 hours at room temperature. The mixture was concentrated in vacuo and purified by reverse phase HPLC (Gilson) to afford the desired product Example 43. 1H NMR (DMSO-d6, 500 MHz) δ 11.46 (s, 1H), 8.76 (s, 1H), 8.12 (m, 1H), 7.94 (m, 1H), 7.41 (m, 1H), 6.87 (m, 1H), 3.43 (m, 2H), 3.26 (m, 2H), 2.89 (m, 2H), 2.18 (m, 1H), 2.11 (m, 2H) 1.88, (m, 1H), 1.30 (m, 3H); LCMS m/z 527 (M+Na). |
|
With pyridine hydrogenfluoride; sodium nitrite; at 0 - 80℃; for 2.75h; |
To a suspension of 5-amino-2-cyano pyridine (20.0 g, 0.168 mol) in HF-pyridine (100 g) in an Erlenmeyer flask cooled to 0 C. was added sodium nitrite (17.4 g, 0.251 mol) in four portions. After 45 min at 0 C. the reaction mixture was stirred at room temperature for 30 min and then heated to 80 C. for 90 min. The reaction mixture was quenched by pouring into ice/water mixture. The resulting mixture was extracted with DCM. The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to give the fluoropyridine as an orange solid. |
|
With sodium nitrite; at 0 - 80℃; for 2.75h; |
EXAMPLE 5; 6 2-[6-FLUORO-3-(2,3 ,6-TRIFLUOROBENZYL)lMIDAZO[ 1 ,5-o]PYRIDIN-l -YL]-5-PYRIDIN-3-YLPYRJMIDIN-4-AMINE; Step A; To a suspension of 5-amino-2-cyano pyridine (20.06 g, 168 mml) in a 2L Erlenmeyer flask in HF-pyridine (lOOg, 3.5 mol) cooled to 0C was added sodium nitrite (17.4 g, 251 mmol) in 4 portions. After 45 minutes at 0C the reaction was stirred at ambient temperature for 30 minutes, and then heated at 80C for 1.5 hours. The reaction mixture was cooled to room temperature and quenched by pouring into ice/water mixture (-400 mL). The resulting solution was extracted with DCM (6X150 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated to yield an orange solid. LC-MS: m/z=T23.09 (M+H); rt=2.32 min (Method A). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping