| 79% |
|
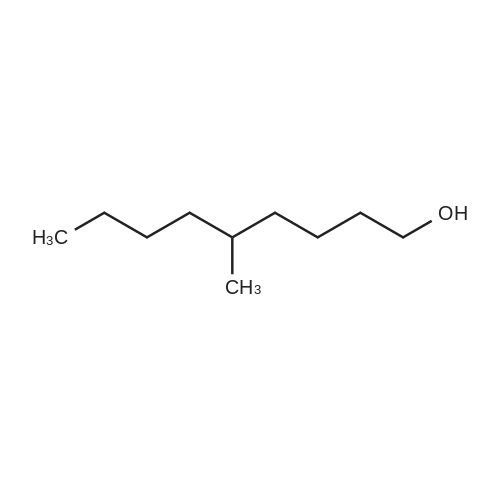
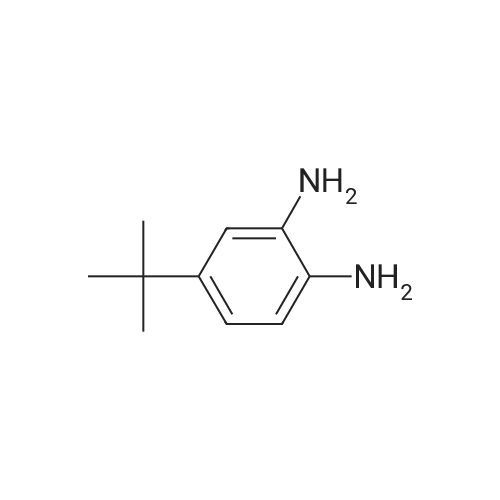
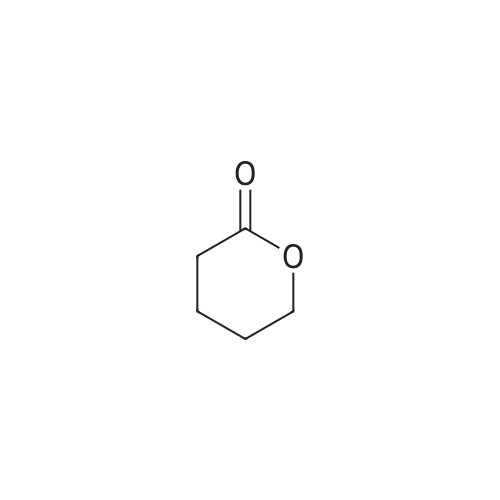
[0553] Step 2: Synthesis of 4-(5-tert-Butyl-lH-benzoimidazol-2-yl)-butan-l-ol[0554] A mixture of 4-tert-butyl -benzene- 1,2-diamine (lg, 6.09mmol) and valerolactone (0.67g, 6.70mmol, l.leq.) in 4N HC1 aq (5ml) was sealed and heated for 2h at 100°C. LCMS analysis shows remaining diamine. Completion was achieved using excess of valerolactone (~1.3ml, 2eq.) heating at 100° for 4h. The reaction was cooled to RT and basified to pH 12 with a saturated solution of Na2C03. EtOAc (100ml) was added and organic separated, washed with saturated solution of Na2C03, water, brine, dried over Na2S04, filtered and concentrated. The residue was purified by flash columnchromatography over silica gel, eluted with DCM - MeOH from 2percent to 5percent gradually to yield 1.19g (79percent) of a beige solid; MS (ESI+) for C15H22N2O m/z 1 [M+H]+, 247.15; HPLC purity 100percent (ret. time, 1.42 min).1H NMR (500 MHz, CHLOROFORM-d) delta ppm 1.38 (9 H, s), 1.65 - 1.75 (2 H, m), 2.00 (2 H, quin, J=7.17 Hz), 2.98 (2 H, t, J=7.09 Hz), 3.72 (2 H, t, J=5.99 Hz), 7.31 (1 H, dd, J=8.51, 1.58 Hz), 7.48 (1 H, d, J=8.20 Hz), 7.56 (1 H, d, J=1.26 Hz). |
| 67% |
|
Compound 319(2R,3R,4S,5R)-2-(6-aminopurin-9-yl)-5-[[4-(5-tert-butyl-lH-benzimidazol-2-yl)butyl- isopropyl-amino]methyl]tetrahydrofuran-3,4-diolHStep 1. Preparation of 4-(5-tert-butyl-lH-benzimidazol-2-yl)butan-l-ol A mixture of 4-tert-butylbenzene-l,2-diamine (646 mg, 3.93 mmol) andtetrahydropyran-2-one (1.18 g, 11.80 mmol) in 50 mL of 4 M HC1 was refluxed for 8 h. Then the mixture was neutralized with K2C03 (aq) to pH = 8. The mixture was extracted with DCM (30 mL x4). The organic layers were concentrated and the residue was purified by SGC (DCM : MeOH = 20 : 1) to afford the product(650 mg, yield: 67 percent) as a pale solid. MS (ESI): m/z 247.7 [M+l]+. |
| 49% |
|
Compound 323(2R^R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5 ((4-(5-(tert-butyl) H-benzo[d]imidazol-2- yl)butyl)thio)methyI)tetrahydrofuran-3,4-diolStep 1. Preparation of 4-(5-tert-butyl-lH-benzimidazol-2-yl)butan-l-olA solution of 4-tert-butylbenzene-l,2-diamine (6 g, 36.59 mmol) andtetrahydropyran-2-one (6.09 g, 60.98 mmol) in 4 M HC1 (100 mL) was heated to 100° C overnight. The reaction was evaporated, added water (50 mL), adjusted to pH = 8 with aq. NaHC03, extracted with EA (3x100 mL), washed with water (20 mL) and brine (20 mL), dried and concentrated. The residue was purified by SGC to obtain the product (4.4 g, Yield 49percent). 1H NMR (500 MHz, MeOD): 57.50-7.28 (m, 3H), 3.60 (t, J = 6.5 Hz, 2H), 2.91 (t, J = 7.5 Hz, 2H), 1.90 (t, J = 7.0 Hz, 2H), 1.60 (t, J = 7.0 Hz, 2H), 1.37 (s, 9H) ppm; ESI-MS (m/z): 247.2[M+1]+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping