| 46% |
With potassium carbonate; In N,N-dimethyl-formamide; at 60℃; |
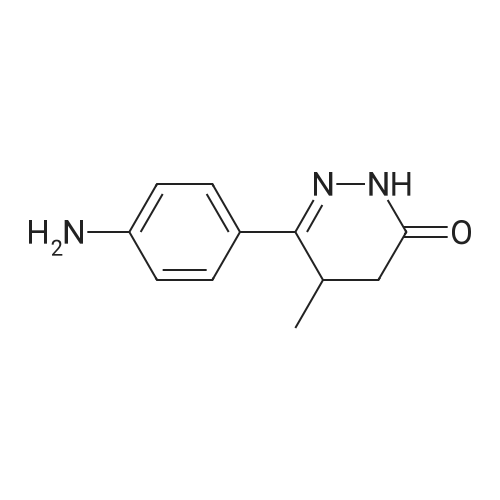
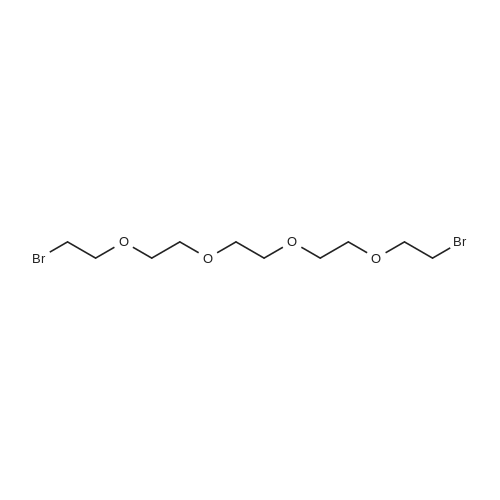
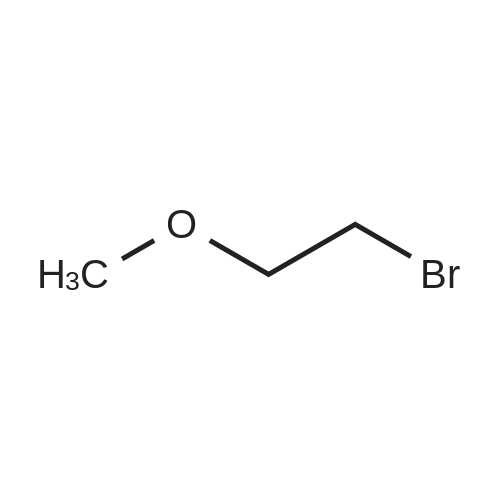
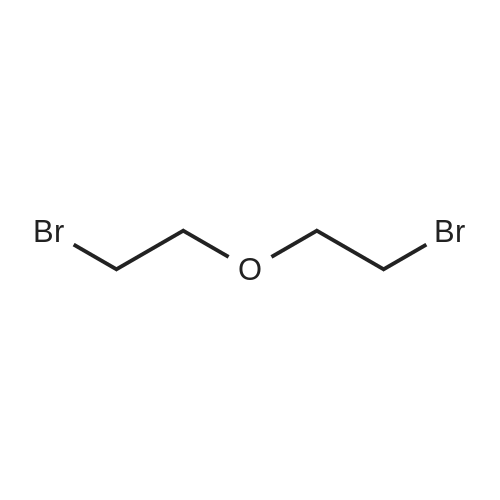
TP5 - To 200 mg (0.984 mmol) of (R)-6-(4-aminophenyl)-5-methyl-4,5- dihydropyridazin-3(2H)-one dissolved in 1 mL of DMF was added 250 (2.00 mmol) of bis (2-bromoethyl) ether and 400 mg of K2CO3 and the mixture was stirred overnight at 60 C. The next day another 250 mu^ of bis (2-bromoethyl) ether and 170 mg of K2CO3 was added. After 3 h, EtOAc and water were added, the water was rinsed with EtOAc, the combined EtOAc washes were dried and concentrated. Chromatography with 0-4% MeOH in CH2C12 yielded 125 mg of product (46%). XH NMR (300 MHz, CDC13) delta 8.61 (s, 1H), 7.68 (d, J= 8.8, 2H), 6.92 (d, J= 8.8, 2H), 3.99 - 3.76 (m, 4H), 3.44 - 3.31 (m, 1H), 3.29 - 3.22 (m, 4H), 2.70 (dd, J= 6.7, 16.8, 1H), 2.46 (d, J= 16.7, 1H), 1.24 (d, J= 7.3, 3H). 13C NMR (75 MHz, CDC13) delta 166.64, 154.05, 152.18, 127.10, 125.33, 1 14.73, 66.69, 48.33, 33.93, 27.94, 16.36. MS: 274 (M + 1). Anal. Calcd. for C15H19N3O2: C, 65.91; H, 7.01 ; N, 15.37; Found. 65.81, H, 6.66, N, 15.26. |
| 46% |
With potassium carbonate; In N,N-dimethyl-formamide; at 60℃;Inert atmosphere; |
To 200 mg (0.984 mmol) of A dissolved in 1 mL of Dimethylformamide (DMF) was added 250 mu^ (2.00 mmol) of bis (2-bromoethyl) ether and 400 mg of K2C03 and the mixture was stirred overnight at 60 C. The next day another 250 mu^ oi bis (2-bromoethyl) ether and 170 mg of K2C03 were added. After 3 hours, EtOAc and water were added, the water was rinsed with EtOAc, the combined EtOAc washes were dried and concentrated. Chromatography with 0-4% MeOH in CH2C12 yielded 125 mg of product (46%). NMR (300 MHz, CDC13) delta 8.61 (s, 1H), 7.68 (d, J = 8.8, 2H), 6.92 (d, J = 8.8, 2H), 3.99 - 3.76 (m, 4H), 3.44 - 3.31 (m, 1H), 3.29 - 3.22 (m, 4H), 2.70 (dd, J = 6.7, 16.8, 1H), 2.46 (d, J = 16.7, 1H), 1.24 (d, J = 7.3, 3H). 13C NMR (75 MHz, CDC13) delta 166.64, 154.05, 152.18, 127.10, 125.33, 114.73, 66.69, 48.33, 33.93, 27.94, 16.36. TLC: Rf 0.1 (1 :50 MeOH:CH2Cl2). HPLC: Rt 1.05 min, purity > 95%. MS: 274 (M + 1). HRMS: calcd. 274.1556 (M + 1); found 274.1552. Anal. Calcd. for G5H19N3O2: C, 65.91 ; H, 7.01; N, 15.37; Found. 65.81, H, 6.66, N, |
| 46% |
With potassium carbonate; In N,N-dimethyl-formamide; at 602℃;Inert atmosphere; |
Step 1 ): (0666) To 200 mg (0.984 mmol) of <strong>[36725-28-7](R)-6-(4-aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one</strong> dissolved in 1 mL of DMF was added 250 muIota_ (2.00 mmol) of bis (2-bromoethyl) ether and 400 mg of K2CO3 and the mixture was stirred overnight at 60 C. The next day another 250 muIota_ of bis (2- bromoethyl) ether and 170 mg of K2CO3 was added. After 3 h, EtOAc and water were added, the water was rinsed with EtOAc, the combined EtOAc washes were dried and concentrated. Chromatography with 0-4% MeOH in CH2C12 yielded 125 mg of product Compound 3 (46%). 1H NMR (300 MHz, CDCI3) delta 8.61 (s, 1 H), 7.68 (d, J = 8.8, 2H), 6.92 (d, J = 8.8, 2H), 3.99 - 3.76 (m, 4H), 3.44 - 3.31 (m, 1 H), 3.29 - 3.22 (m, 4H), 2.70 (dd, J = 6.7, 16.8, 1 H), 2.46 (d, J = 16.7, 1 H), 1 .24 (d, J = 7.3, 3H). 13C NMR (75 MHz, CDCI3) delta 1 66.64, 154.05, 152.18, 127.1 0, 125.33, 1 14.73, 66.69, 48.33, 33.93, 27.94, 1 6.36. MS: 274 (M + 1 ). Anal. Calcd. for C15H19N3O2: C, 65.91 ; H, 7.01 ; N, 15.37; Found. 65.81 , H, 6.66, N, 15.26. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping