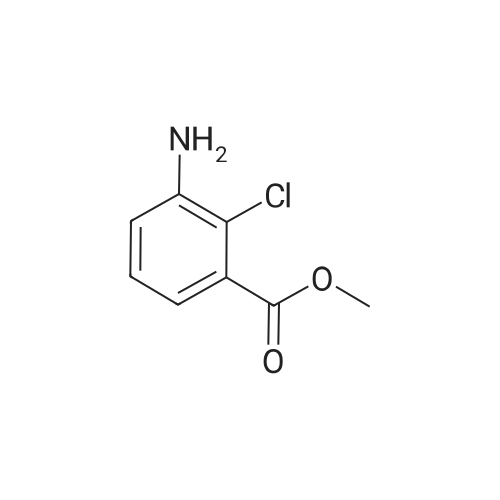
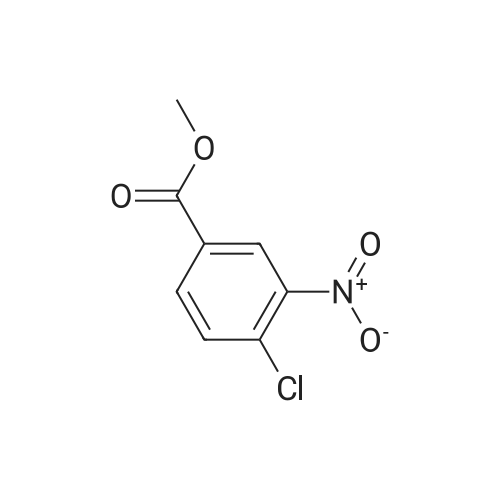
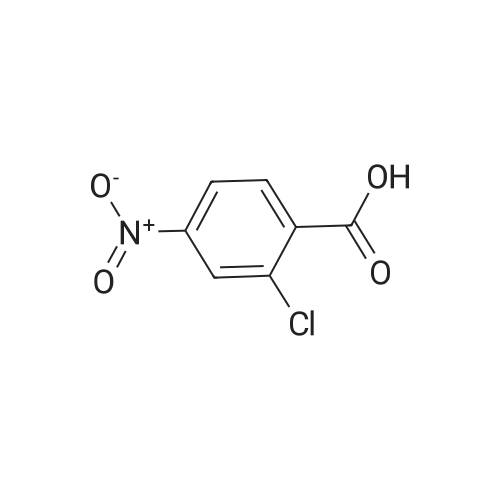
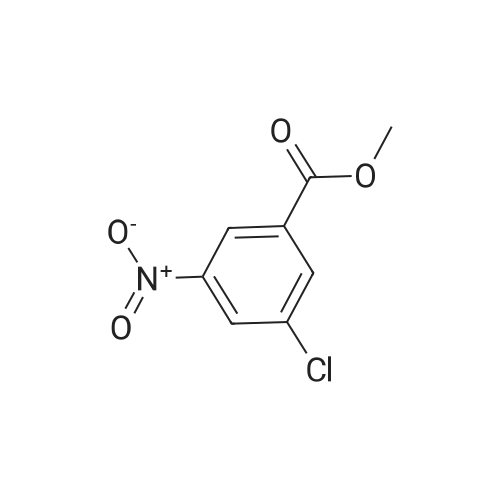
| 83.5% |
With hydrogen;nickel; In methanol; at 25℃; under 2585.81 Torr; for 3.5h; |
Step B: Methyl 3-amino-2-chlorobenzoate; To a solution of methyl 2-chloro-3-nitrobenzoate (25 g, 116 mmol) in MeOH (150 mL) was added Raney Ni (3 g). The mixture was stirred under H2 atmosphere (50 psi at 25 C.) for 3.5 h. The catalyst was filtered, and the filtrate was concentrated under the reduced pressure to dryness to give the crude product, which was purified by recrystallization in EtOAc to afford the title compound (69 g, 83.5% yield, four batches combined). 1H NMR (400 MHz, CDCl3) delta ppm 6.70-7.25 (m, 3H), 4.40-4.50 (br, 2H), 3.87 (s, 3H). |
| 2.6 g (50%) |
With sodium hydroxide; stannous chloride; In hydrogenchloride; ethanol; |
b) Methyl 2-chloro-3-aminobenzoate A solution of stannous chloride (27.0 g, 137.0 mM) in 55 mL of concentrated hydrochloric acid was slowly added to a solution of methyl 2-chloro-3-nitrobenzoate (6.0 g, 27.9 mM) in 75 mL ethanol at 15-20 C. over 1 hour. The mixture was then heated at 50-60 C. for 15 minutes. The mixture was cooled to room temperature and made alkaline by slow addition of solid sodium hydroxide maintaining a temperature of 30-35 C. The resultant mixture was extracted three times with chloroform. The extracts were washed with brine, dried over sodium sulfate, filtered and concentrated to afford 2.6 g (50%) of methyl 2-chloro-3-aminobenzoate as a yellow oil, identical in all respects to the material derived via catalytic hydrogenation described above. |
| 1.2 g (33%) |
With sodium carbonate; In tetrahydrofuran; methanol; water; |
b) Methyl 2-chloro-3-aminobenzoate A solution of sodium dithionite (14.0 g, 20.0 mM) and sodium carbonate (6.7 g) in 200 mL of water was slowly added to a solution of methyl 2-chloro-3-nitrobenzoate (6.0 g, 27.9 mM) in 40 mL methanol and 40 mL of tetrahydrofuran at 25 C. over 30 minutes. The mixture was stirred at room temperature for an additional 30 minutes, then extracted with ethyl acetate. The extracts were washed with brine, dried over sodium sulfate, filtered and concentrated to afford 1.2 g (33%) of methyl 2-chloro-3-aminobenzoate as a yellow oil, identical in all respects to the material derived via catalytic hydrogenation described above. |
|
With ammonium formate; acetic acid; zinc; In tetrahydrofuran; methanol; at 20℃; for 18h; |
Step A: methyl 3-amino-2-chlorobenzoate: To a solution of methyl 2-chloro-3-nitrobenzoate (2.1 g, 9.7 mmol) in methanol (100 mL) and THF (20 mL) was added zinc powder (1.9 g, 29 mmol), ammonium formate (3.1 g, 49 mmol), and a few drops of acetic acid. The mixture was allowed to stir at RT for 18 hours. Most of the volatiles were removed under reduced pressure. The residue was redissolved in EtOAc (200 mL), washed with brine, concentrated and purified by MPLC to provide methyl 3-amino-2-chlorobenzoate. |
|
With ammonium formate; acetic acid; zinc; In tetrahydrofuran; methanol; at 20℃; for 18h; |
Step A: methyl 3-amino-2-chlorobenzoate: To a solution of methyl 2-chloro-3-nitrobenzoate (2.1 g, 9.7 mmol) in methanol (100 mL) and THF (20 mL) was added zinc powder (1.9 g, 29 mmol), ammonium formate (3.1 g, 49 mmol), and a few drops of acetic acid. The mixture was allowed to stir at RT for 18 hours. Most of the volatiles were removed under reduced pressure. The residue was redissolved in EtOAc (200 mL), washed with brine, concentrated and purified by MPLC to provide methyl 3-amino-2-chlorobenzoate. |
| 19 g |
With iron; ammonium chloride; In methanol; water; at 80℃; for 2h; |
To a solution of methyl 2-chloro-3-nitrobenzoate (23.0 g, 107 mmol) in MeOH (100 mL) and H2O (20 mL) were added Fe (29.8 g, 533 mmol) and NH4Cl (45.7 g, 853 mmol). The mixture was stirred at 80 C for 2 hours. The mixture was filtered out and the filtrate was concentrated to give a residue. The residue was purified by silica gel chromatography to give methyl 3-amino-2- chlorobenzoate (I-484) (19.0 g) as a yellow oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping