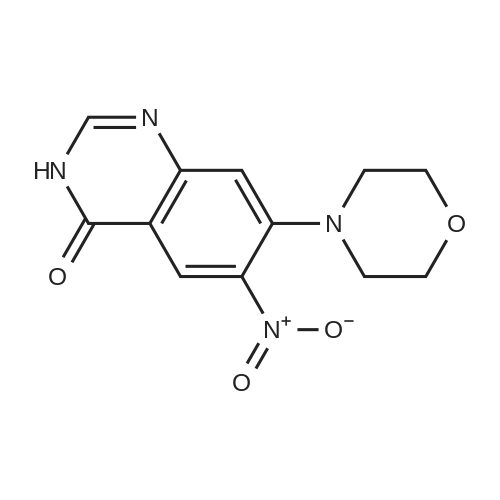
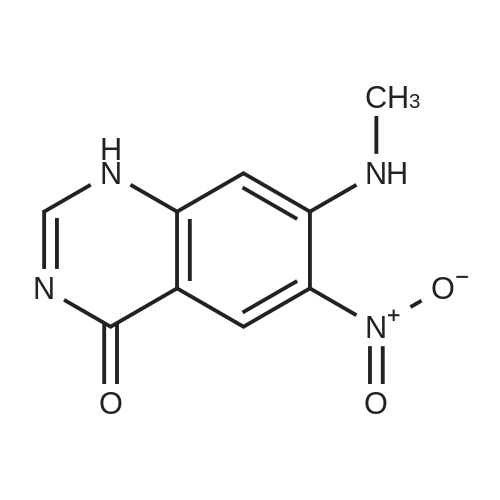
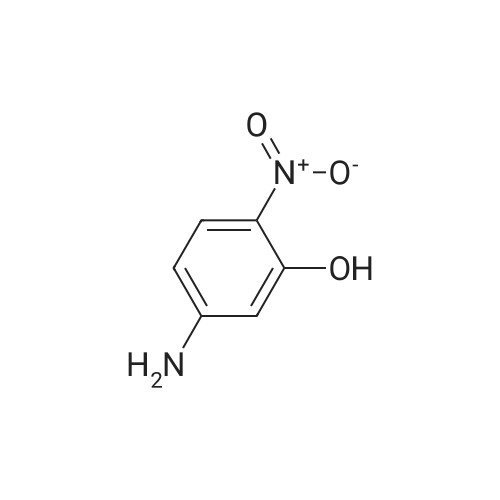
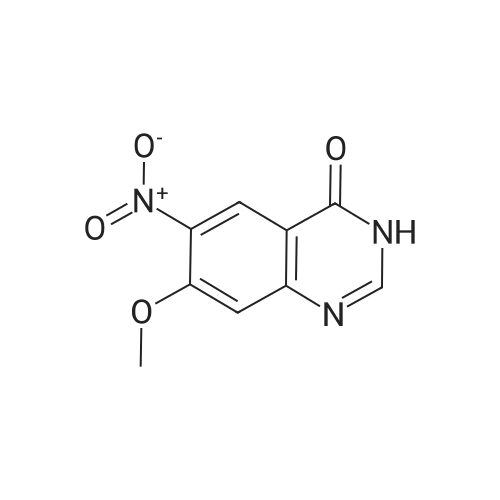
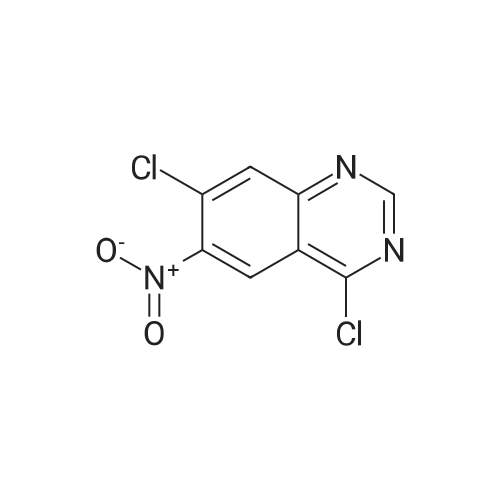
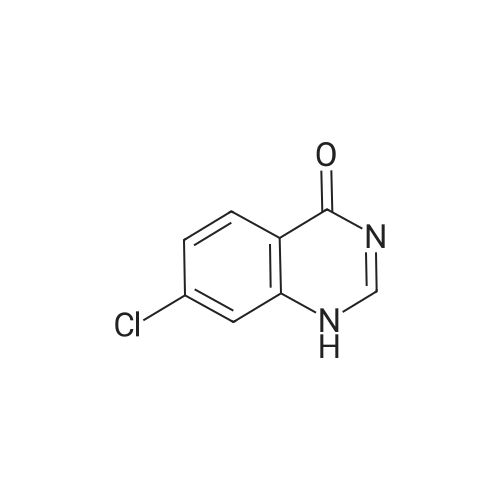
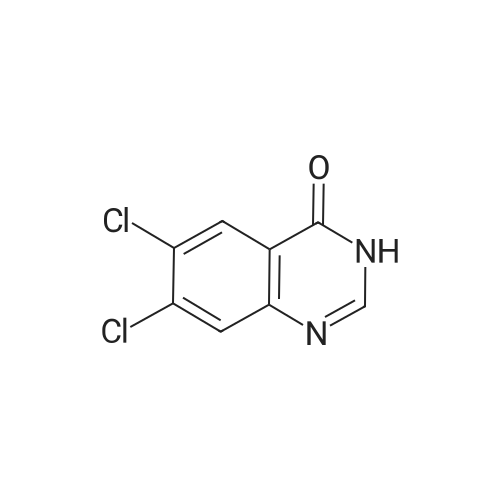
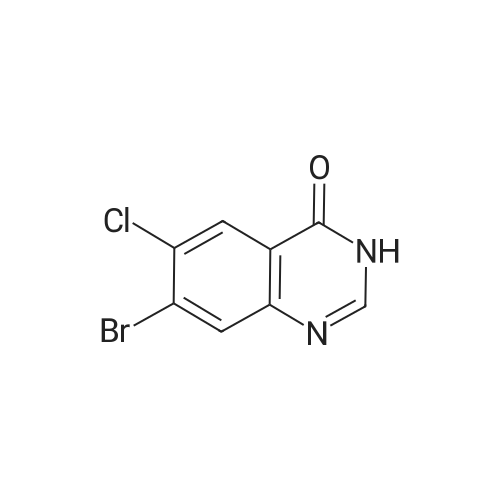
| 62.7% |
With sulfuric acid; nitric acid; In water; at 0 - 45℃; |
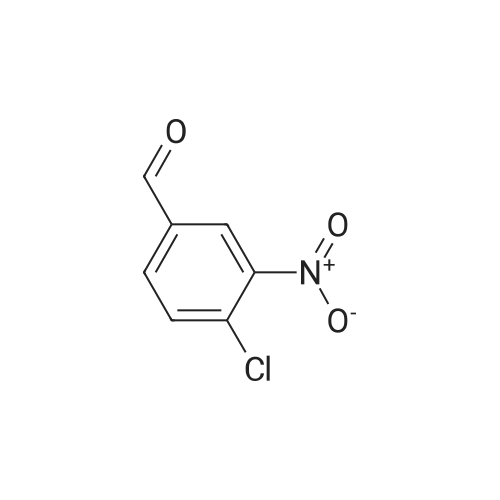
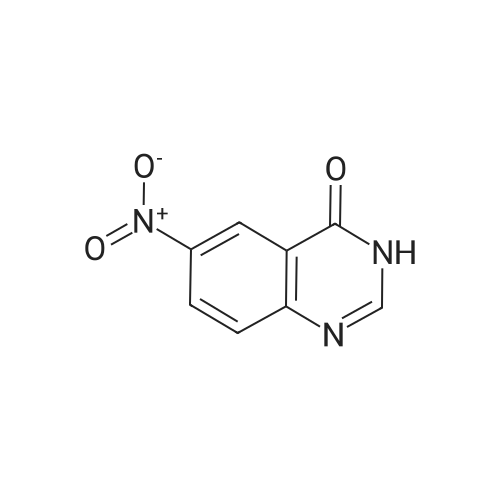
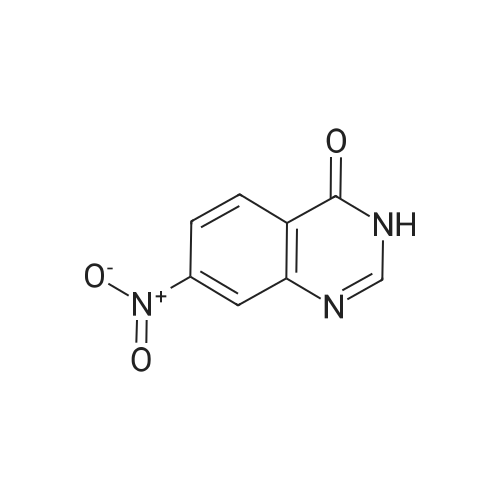
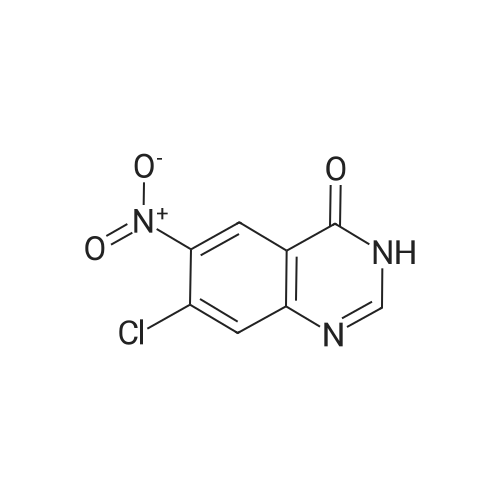
Compound 0302 (18.0 g, 100 mmol) was added portionwise to a stirred mixture of concentrated sulfuric acid (60 mL) and fuming nitric acid (60 mL) which had been cooled to 0 0C, the mixture was stirred at ambient temperature for 1 hour and then heated to 45 0C overnight. The mixture was poured into the mixture of ice and water. The precipitate was isolated, washed with water and dried. Recrystallization from acetic acid to give the title compound 0303 (14.1 g, 62.7%). 1H NMR (DMSO-J6): delta 8.00 (s, IH), 8.27 (s, IH), 8.65 (s, IH), 12.70 (s, IH). |
| 62.7% |
With sulfuric acid; nitric acid; at 0 - 45℃; |
Step 22b. 7-Chloro-6-nitroquinazolin-4(3H)-one (compound 0303) Compound 0302 (18.0 g, 100 mmol) was added portionwise to a stirred mixture of concentrated sulfuric acid (60 mL) and fuming nitric acid (60 mL) which had been cooled to 0 C., the mixture was stirred at ambient temperature for 1 hour and then heated to 45 C. overnight. The mixture was poured into the mixture of ice and water. The precipitate was isolated, washed with water and dried. Recrystallization from acetic acid to give the title compound 0303 (14.1 g, 62.7%). 1H NMR (DMSO-d6): delta 8.00 (s, 1H), 8.27 (s, 1H), 8.65 (s, 1H), 12.70 (s, 1H). |
| 62.7% |
With sulfuric acid; nitric acid; at 0 - 45℃; |
Step 22b. 7-Chloro-6-nitroquinazolin-4(3H)-one (compound 0303); Compound 0302 (18.0 g, 100 mmol) was added portionwise to a stirred mixture of concentrated sulfuric acid (60 mL) and fuming nitric acid (60 mL) which had been cooled to 0 C., the mixture was stirred at ambient temperature for 1 hour and then heated to 45 C. overnight. The mixture was poured into the mixture of ice and water. The precipitate was isolated, washed with water and dried. Recrystallization from acetic acid to give the title compound 0303 (14.1 g, 62.7%). 1H NMR (DMSO-d6): delta 8.00 (s, 1H), 8.27 (s, 1H), 8.65 (s, 1H), 12.70 (s, 1H). |
|
With sulfuric acid; nitric acid; |
Reference example 3: 7-chloro-6-nitro-4(3H)-quinazolone 7-Chloro-4(3H)-quinazolone (20.9 g, 0.116 mmol) obtained in Reference example 2 was dissolved in a mixed solution of concentrated sulfuric acid (45 ml) and fuming nitric acid (45 ml) and stirred at 80 C for 2 hours. After completion of the reaction, the reaction solution was cooled to room temperature and poured into ice water (900 ml). The precipitated solid was filtered off, and the filtered crude crystal was suspended in acetic acid (440 ml) and stirred at 80 C for 1 hour. |
|
With sulfuric acid; nitric acid; at 0 - 90℃; for 3h; |
10g of the above 7-chloro-quinazolone was added into a mixed acid of concentrated sulphuric acid and fuming nitric acid (40ml) slowly in an ice-bath. Then the mixture was heated to 90 and reacted at this temperature for 3h. The clear solution formed was then poured into 300mL of ice-water carefully, and yellow solid was deposited, which was filtered, washed with water and redissolved into hot acetic acid, to deposit the crystalline of 6-nitro-7-chloro-quinazolone, which was collected and 6.50g of the product was achieved. |
|
With sulfuric acid; nitric acid; at 0 - 90℃; for 3h; |
10 g of the above 7-chloro-quinazolone was added into a mixed acid of concentrated sulphuric acid and fuming nitric acid (40 ml) slowly in an ice-bath. Then the mixture was heated to 90 C. and reacted at this temperature for 3 h. The clear solution formed was then poured into 300 mL of ice-water carefully, and yellow solid was deposited, which was filtered, washed with water and redissolved into hot acetic acid, to deposit the crystalline of 6-nitro-7-chloro-quinazolone, which was collected and 6.50 g of the product was achieved. |
|
With sulfuric acid; nitric acid; at 90℃; for 1h;Cooling with ice; |
The compound BB2 under 10g ice bath was slowly added concentrated sulfuric acid and fuming nitric acid (20ml: 20ml) mixed acid, the addition is complete warm to 90 C, for about 1 hour. The reaction solution was poured into 300ml of ice water, the precipitated solid was collected by filtration and the solid was the crude compound 12g BB3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping