|
With manganese(IV) oxide; In dichloromethane; at 25℃; for 68h; |
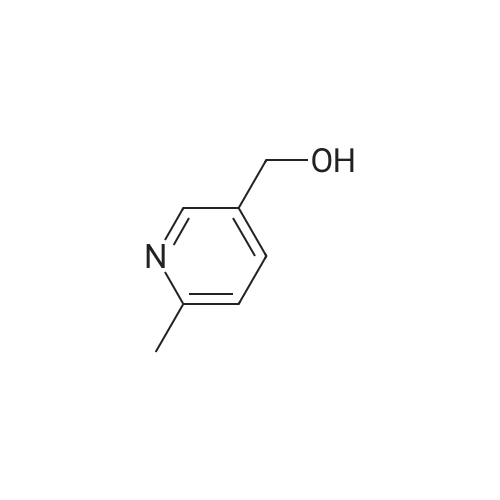
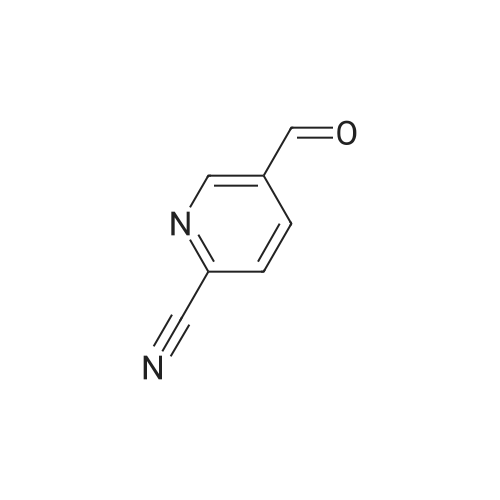
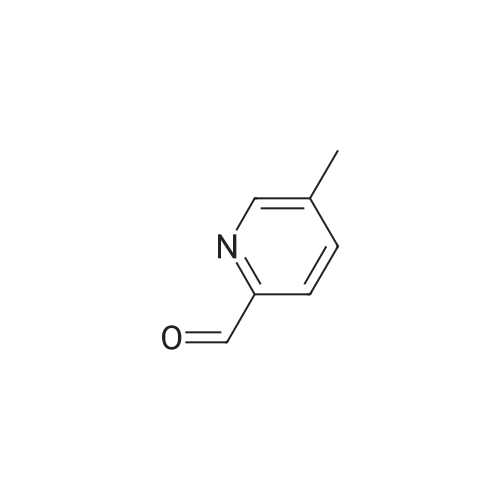
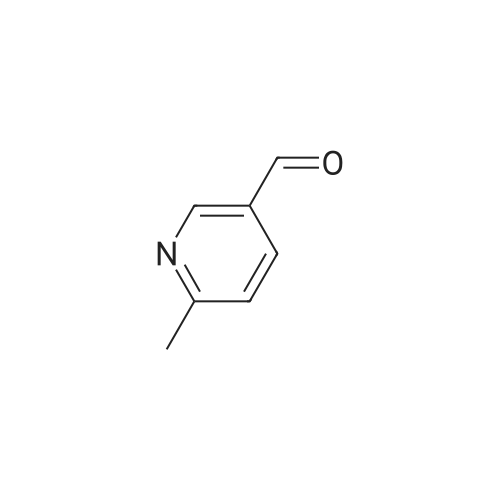
A solution of methyl 6-methylnicotinate (0.5 g, 3.3 mmol) in THF (16 mL) at 0 C. was treated dropwise with lithium aluminum hydride in THF (6.6 mL, 1 M), stirred at 0 C. for 1.5 hours, treated with ethyl acetate (3 mL), stirred at 25 C. The reaction was partitioned between ethyl acetate and saturated NaHCO3, and the organic phase was washed with brine and dried over MgSO4, filtered and concentrated. A solution of the residue (0.395 g) in dichloromethane (16 mL) was treated with MnO2 (2 g), stirred at 25 C. for 68 hours, filtered through celite to give the title compound (0.326 g, 80% yield), which was used without further purification. |
|
In pyridine; water; |
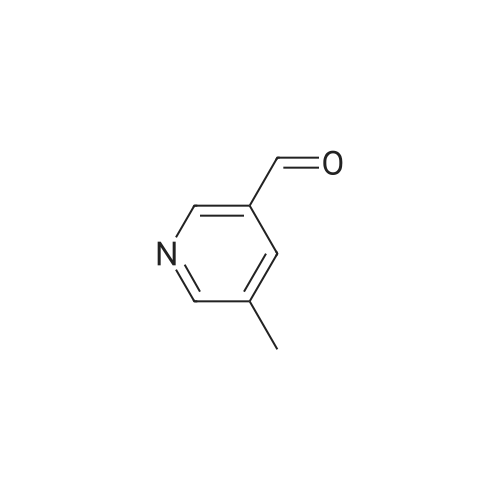
Chromium trioxide (11.5 g) is slowly added to 170 ml of pyridine at 20C, and 10 g of the crude 5-hydroxy-methyl-2-methylpyridine in 70 ml of pyridine is added in one portion to the complex. The temperature is raised to reflux temperature for 2 hours, and the mixture is refluxed for 1.5 hours. After cooling, 250 ml of water is added, and the mixture is extracted with five 150-ml portions of diethyl ether. The combined extracts are dried over magnesium sulfate and concentrated to give 4.2 g of crude 6-methyl-3-pyridinecarbaldehyde. |
|
With manganese(IV) oxide; In dichloromethane; at 0 - 20℃; for 1.25h; |
Stage 2 - Preparation of 6-methylnicotinaldehyde; To a cooled (ice bath) solution of stage 1 product (14.85g, 120.6mmol) in DCM (100OmL) was added manganese oxide (10Og) in 1Og portions over a period of 30 minutes. The mixture was warmed to RT and stirred for 45 minutes and was then filtered through Celite. The residues were washed with DCM (50OmL), and the combined DCM portions were concentrated to yield the desired product. 1 H NMR (300MHz, CDCI3) delta: 10.00 (1H, s), 8.88 (1 H, d, J=1.8Hz), 8.00 (1 H, dd, J=2.4, 8.1 Hz), 7.26 (1 H, d, J=8.1 Hz), 2.60 (3H, s). |
|
|
To a stirred solution of dimethyl sulfoxide (25.3 mL, 0.357 mol) and CH2Cl2 (600 mL) under nitrogen at -78 0C was slowly added oxalyl chloride (16 mL, 0.19 mol). After completion of the addition, the mixture was stirred for additional 10 min. To the resulting solution was added dropwise a solution of (6-methyl-3-pyridyl)methanol (20 g, 0.162 mol) in CH2Cl2 (10 mL), and then the mixture was stirred at -78 0C for 2.5 hr. Triethylamine (110 mL, 0.82 mol) was slowly added at -78 0C and then the mixture was slowly warmed to room temperature and stirred for another 1 hr. The mixture was treated with water and the aqueous phase was extracted with CH2Cl2 (3 x 500 mL). The combined organic layers were washed with brine, dried over anhydrous MgSO4, filtered and concentrated. The residue was purified by silica gel column to afford the title compound. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping