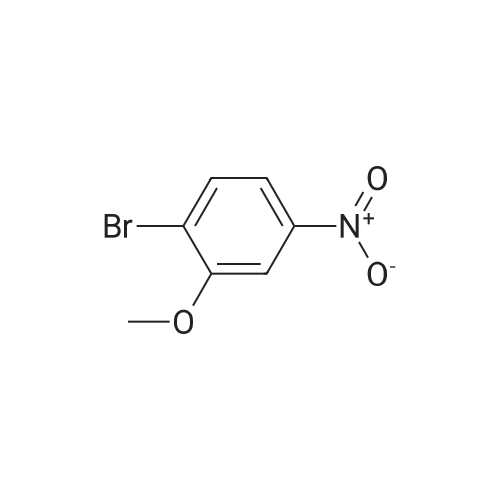
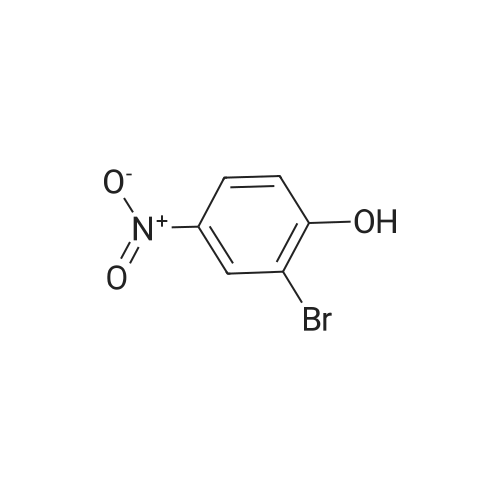
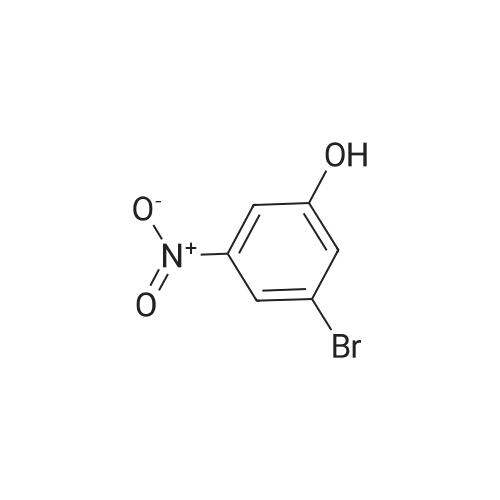
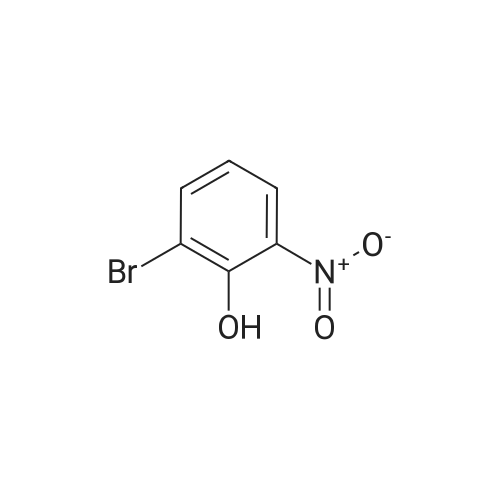
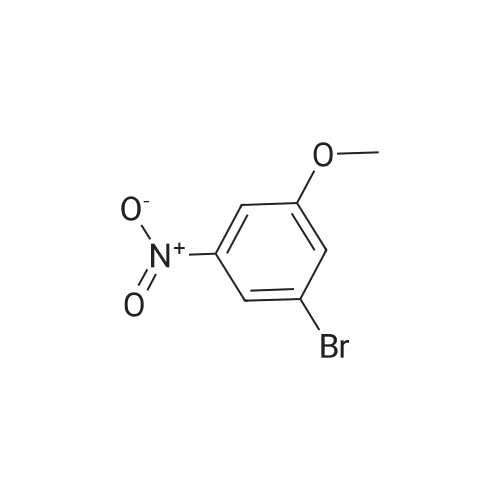
| 78% |
|
To a 500 niL round bottom flask charged with 2-bromo-5-nitroanisole (11.0 g, 47 mmol) was added 100 mL of anhydrous DCM. Aluminum chloride (25 g, 150 mmol) was then added to the reaction mixture. The resulting suspension was heated overnight under nitrogen at 50 0C. The reaction was allowed to cool to rt, poured over ice, and acidified to pH 4 with the addition of aqueous 10 percent HCl. The resulting mixture was filtered through a bed of Celite and the filtrate was transferred to a separatory funnel. The aqueous phase was extracted with methylene chloride (~ 2 x 200 mL). The combined organic phase was dried over Na2SO4 and cone in vacuo giving the crude title compound which was purified by silica gel chromatography (330 g) using EtOAc/hexanes (1 : 1) as eluent to afford the title compound (8.0 g, 78 percent) m/z: 217 |
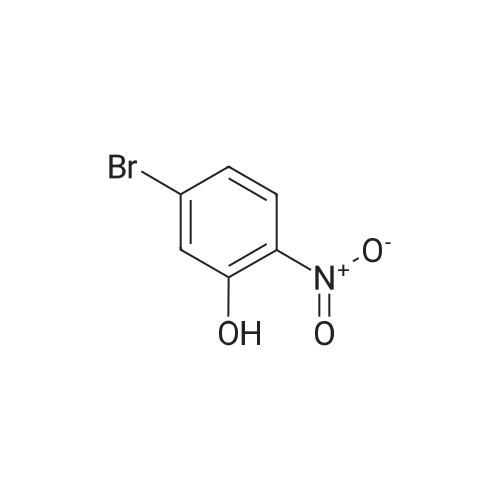
| 71% |
|
To a solution of l-bromo-2-methoxy-4-nitrobenzene (3 g, 12.93 mmol) in CH2CI2 (5 niL) at -78 °C was added tribromoborane (38.8 niL, 38.8 mmol). The resulting mixture was stirred at room temperature overnight and then diluted with EtOAc and water. The separated organic layer was washed with brine, dried over sodium sulfate and concentrated. The residue was purified by flash chromatography on silica to yield 16A (2.0 g, 71percent yield). MS (ES): m/z= 218.0 [M+H]+. 3/4 NMR (500 MHz, DMSO-d6) delta ppm 7.55-7.60 (m, 1 H) 7.61-7.66 (m, 1 H) 7.79 (d, J=2.20 Hz, 1 H). |
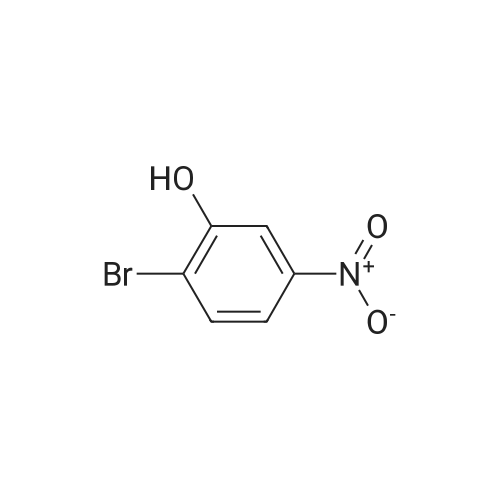
| 65% |
With boron tribromide; In dichloromethane; at 0 - 20℃; |
BBr3 (8.58 mL of 1.0M solution, 8.6 mmol) was added to the DCM (10 mL) solution of 1-bromo-2-methoxy-4-nitrobenzene (1.0 g, 4.3 mmol) at 0° C. The reaction mixture was warmed to room temperature and stirred overnight. The mixture was quenched with water and extracted with ethyl acetate (3×20 mL). The combined extracts were dried and the crude was purified by flash column chromatography to get the desired compound as pale brown powder in 65percent yield (600 mg). [0227] 1H NMR (400 MHz, CDCl3) delta 7.84 (s, 1H), 7.79-7.59 (m, 2H), 5.92 (br s, 1H). [0228] 13C NMR (101 MHz, CDCl3) delta 152.97, 148.49, 132.62, 117.44, 116.43, 111.20. |
| 63% |
With hydrogen bromide; In water; for 20h;Reflux; |
INTERMEDIATE PREPARATION 164-bromo-3-[ -methylethyl)oxy]aniline2-bromo-5-nitrophenolA mixture of 2-bromo-5-nitrophenyl methyl ether (5 g, 2.17 mmol) in HBr (10 mL) was heated to reflux for 20 h. The reaction mixture was poured into ice cold water (100 mL) and filtered. The solid was collected by filtration, dissolved in ethyl acetate (50 mL), dried over Na2S04, filtered, and concentrated in vacuo. The crude product was purified via column chromatography (30:1-20:1 petroleum ether/ethyl acetate) to afford 2-bromo-5-nitrophenol (9.49 g, 63percent yield) as a yellow solid |
| 27.4 g (125.6 mmol, 100%) |
With boron tribromide; In dichloromethane; |
Step 1 2-Bromo-5-nitrophenol A mixture of 29.1 g (125.6 mmol) of 2-bromo-5-nitroanisole and 250 mL CH2Cl2 was stirred at -44° C. in a dry ice acetonitrile bath under a nitrogen atmosphere. Boron tribromide (18 mL) was added dropwise to the reaction mixture. The resulting black reaction was stirred while allowing the cooling bath to slowly rise to room temperature over 20 h. TLC analysis indicated complete reaction, so the reaction mixture was transferred to an addition funnel and added cautiously to a mixture of ice, water and 75 g solid KH2PO4. The layers were separated, the aqueous layer was washed with CH2Cl2, the combined organic extracts were washed with brine, dried over anhydrous magnesium sulfate, filtered, concentrated to give 27.4 g (125.6 mmol, 100percent) of 2-bromo-5-nitrophenol as a black solid. 1H NMR (400 MHz) CDCl3 7.85 (s, 1H), 7.62-7.70 (m, 2H), 5.88 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping