| 78% |
|
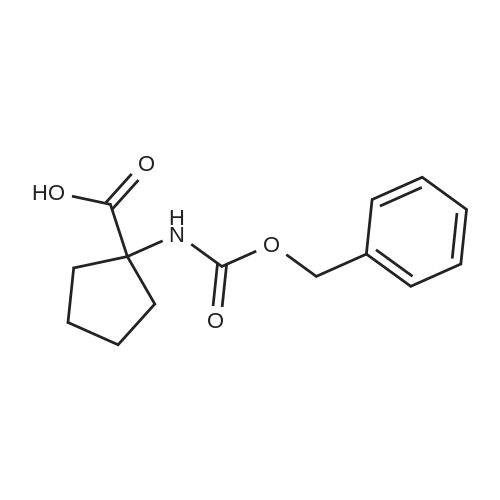
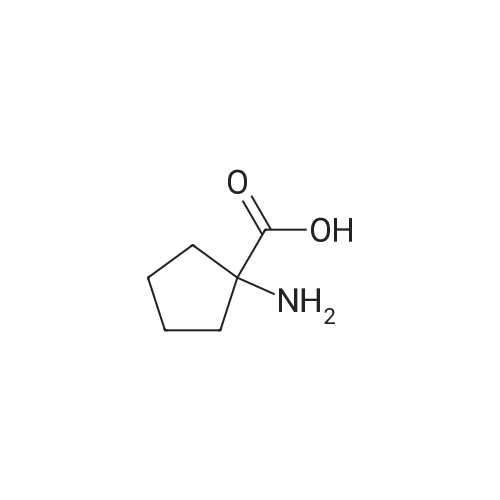
To a solution of 1-aminocyclopentanecarboxylic acid (3.0 g, 23.2 mmol) in 1:1 dioxane/water (60 mL), was slowly added Na2CO3 (12.3 g, 116 mmol) followed by benzyl chloroformate (3.6 mL, 25.5 mmol) and the mixture stirred overnight at RT. The reaction mixture was carefully acidified to pH=2 with 1M HCl then extracted with EtOAc (3*30 mL). The combined organic extracts were washed with brine (30 mL), dried (MgSO4), filtered and concentrated in vacuo to leave a pale yellow oil. LCMS and NMR showed the crude product to be a mixture of desired product and corresponding benzyl ester. The crude product was dissolved in 1:1 THF/water (60 mL) and treated with lithium hydroxide (2.67 g, 116 mmol). The mixture was stirred at RT overnight then washed with Et2O (3*30 mL), acidified to pH=2 and extracted with EtOAc (3*30 mL). The combined organic extracts were washed with brine (30 mL), dried (MgSO4), filtered and concentrated under reduced pressure to afford the title compound (4.76 g, 78%). LCMS: m/z 264 [M+H]+. |
| 78% |
|
To a solution of 1-aminocyclopentanecarboxylic acid (3.0 g, 23.2 mmol) in 1 :1 dioxane / water (60 ml), was slowly added Na2CO3 (12.3 g, 116 mmol) followed by benzyl chloroformate (3.6 ml, 25.5 mmol) and the mixture stirred overnight at RT. The reaction mixture was carefully acidified to pH 2 with 1 M HCI then extracted with EtOAc (3 x 30 ml). The combined organic extracts were washed with brine (30 ml), dried (MgSO4), filtered and concentrated in vacuo to leave a pale yellow oil. LCMS and NMR showed the crude product to be a mixture of desired product and corresponding benzyl ester. The crude product was dissolved in 1 :1 THF / water (60 ml) and treated with lithium hydroxide (2.67 g, 116 mmol). The mixture was stirred at RT over night then washed with Et2O (3 x 30 ml), acidified to pH 2 and extracted with EtOAc (3 x 30 ml). The combined organic extracts were washed with brine (30 ml), dried (MgSO4), filtered and concentrated under reduced pressure to afford the title compound (4.76 g, 78%). LCMS: m/z 264 [M+H]+. |
| 62% |
With sodium carbonate; In 1,4-dioxane; water; at 0 - 20℃; |
A solution of benzyl chloroformate (0.290 g, 1.1 mmol) in dioxane (2.5 cm3) was added dropwise to a solution of 1 -aminocyclopentanecarboxylic acid (Fluka) (0.2 g, 1.54 mmol) and sodium carbonate (0.490 g, 4.64 mmol) in water (5 cm3) at 0 C. Stirring was continued at room temperature overnight and the reaction mixture washed with ether. The aqueous layer was acidified with 2M hydrochloric acid, extracted with ethyl acetate, dried (Na2SO4), filtered and the solvent removed to afford carbamate 21 (0.253 g, 62%) as an oil which solidified on standing. Carbamate 21 was shown to be a 70:30 mixture of conformers by 1H NMR analysis (the ratio was estimated from the integration of the resonances at delta 5.31 and 7.29-7.40, assigned to the N-H protons of the major and minor conformers, respectively): mp 70-80 C (lit.1 82-86 C, ethyl acetate, petroleum ether); SH (400 MHz; CDCl3; Me4Si) 1.83 (4H, br s, 2 x cyclopentyl-H2), 2.04 (2H, br s, cyclopentyl-H2), 2.20-2.40 (2H, m, cyclopentyl- H2), 5.13 (2H, br s, OCH2Ph), 5.31 (0.7eta, br s, N-H) and 7.29-7.40 (5.3H, m, Ph and N-H*); deltac (100 MHz; CDCl3) 24.6 (CH2, cyclopentyl-C), 37.5 (CH2, cyclopentyl-C),' denotes resonance assigned to minor conformer. <n="31"/>66.0 (quat., cyclopentyl-C), 66.8 (CH2, OCH2Ph), 128.0 (CH, Ph), 128.1 (CH, Ph), 128.4 (CH, Ph), 136.1 (quat, Ph), 155.8 (quat., NCO2) and 179.5 (quat., CO2H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping