| 83% |
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20℃; |
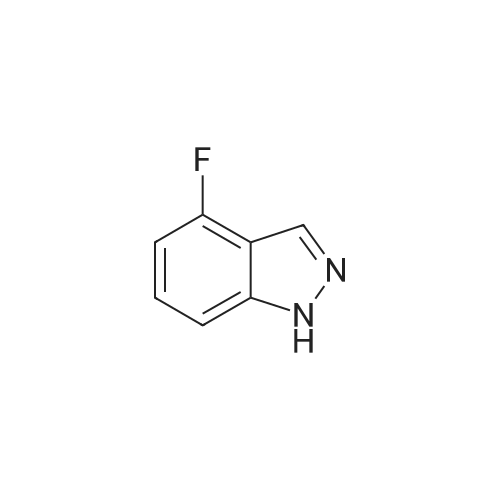
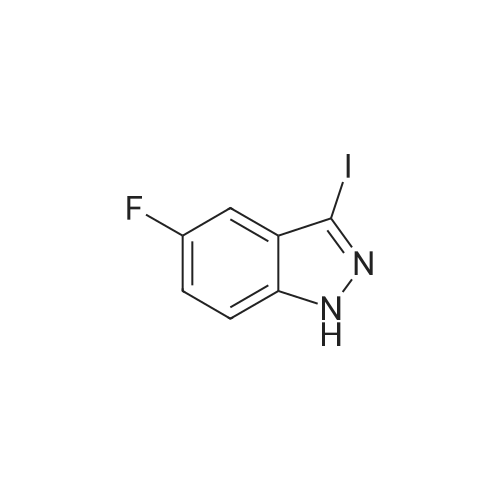
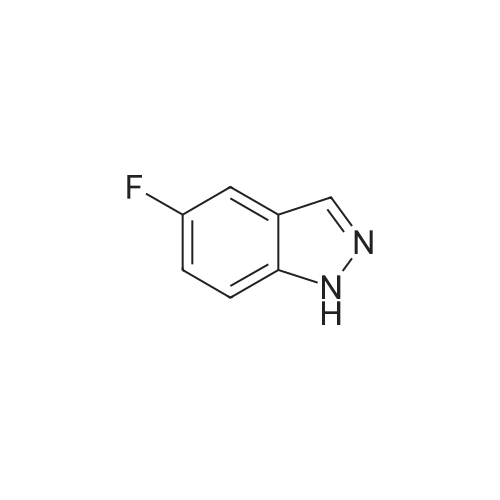
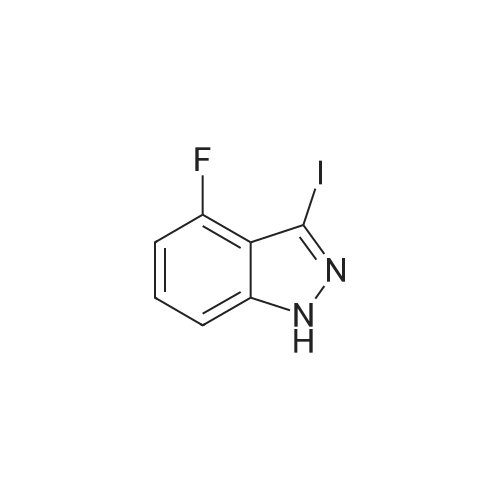
A suspension of <strong>[341-23-1]4-fluoro-1H-indazole</strong> (500 mg, 3.67 mmol, 1.00 equiv), iodine (1.87 g, 2.00 equiv) and potassium hydroxide (741 mg, 13.21 mmol, 3.60 equiv) in N,N-dimethylformamide (5mL) was stirred overnight at room temperature. The reaction was quenched by 10% aqueous NaHSO3, extracted with ethyl acetate, washed with brine, dried over anhydrous sodium sulfate, and concentrated under vacuum. The resulting solid was washed with petroleum ether to give 800 mg (83%) of the title compound as a yellow solid. LC-MS (ES, m/z): 263 [M+H]. |
| 65% |
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 0 - 20℃; |
To a solution of 4-fluoro-lH-indazole (i-3a) (24 g, 180 mmol) in DMF (300ml) was added iodine (56 g, 216 mmol) and KOH (40 g, 720 mmol) at 0C. The resultant mixture was allowed to warm to room temperature and stirred for 5 h. The reaction mixture was slowly quenched with saturated sodium thiosulfate (200 mL) and extracted with EtOAc (500 mL x 3). The combined organic layers were washed, dried and concentrated, and the residue was purified by re-crystallization to afford the title compound (30 g, yield: 65%). LCMS (ESI) calc'd for C7H4FIN2 [M+H]+: 263, found: 263. |
| 65% |
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 0 - 20℃; for 5h; |
Step 1. Preparation of 4-fluoro-3-iodo-1H-indazole (i-7a).To a solution of <strong>[341-23-1]4-fluoro-1H-indazole</strong> (24 g, 180 mmol) in 300 mL of DMF was added diiodine (56 g, 216 mmol) and potassium hydroxide (40 g, 720 mmol) at 0 C. The resultant mixture was allowed to warm to room temperature and stirred for 5 hours. The reaction mixture was slowly quenched with saturated sodium thiosulfate (200 mL) and extracted with EA (500 mL * 3), and the combined organic layers were washed, dried and concentrated. Theresidue was purified by re-crystallization to afford the title compound (30 g, yield: 65%). LCMS (ESI) calc?d for C7H4FIN2 [M+H]: 263, found: 263. |
| 65% |
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 0℃; for 20h; |
i). Preparation of 4-fluoro-3-iodo-1H-indazole (i-3b) To a solution of <strong>[341-23-1]4-fluoro-1H-indazole</strong> (i-3a) (24 g, 180 mmol) in DMF (300 ml) was added iodine (56 g, 216 mmol) and KOH (40 g, 720 mmol) at 0 C. The resultant mixture was allowed to warm to room temperature and stirred for 5 h. The reaction mixture was slowly quenched with saturated sodium thiosulfate (200 mL) and extracted with EtOAc (500 mL*3). The combined organic layers were washed, dried and concentrated, and the residue was purified by re-crystallization to afford the title compound (30 g, yield: 65%). LCMS (ESI) calc'd for C7H4FIN2 [M+H]+: 263. found: 263. |
|
With iodine; potassium hydroxide; In N,N-dimethyl-formamide; at 20℃; for 2h; |
Example 24A: Preparation of sodium 4-(l-(2-chloro-6-(trifluoromethyl) benzoyl)-4- fluoro-/H-indazol-3-yl)-3-fluorobenzoate (24A)A-5 A-6 24A i) Preparation of 4-fluoro-3-iodo-/H-indazole (A-2). To a solution of <strong>[341-23-1]4-fluoroindazole</strong> A-l (5.00 g, 36.7 mmol) in DMF (80 mL) was added h (18.6 g, 73.5 mmol) and KOH (7.73 g, 134 mmol) successively at rt. After 2 h, the reaction mixture was poured into aq. 10% NaHS03 (200 mL) and extracted with EtOAc (200 mL*3). The combined organic layers were washed with H20 and brine, dried over Na2S04, and concentrated. The crude solid was washed with PE to give the title compound as a yellow solid. LCMS (ESI) calc'd for C7H5FIN2 [M+H]+: 262.9, found: 262.9 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping