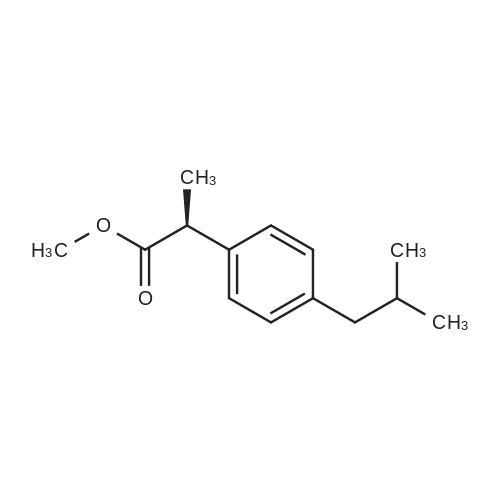
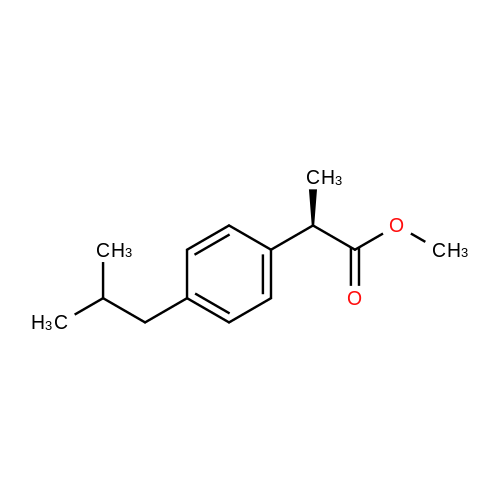
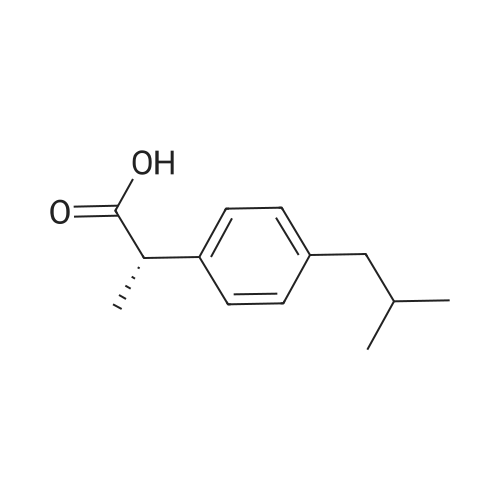
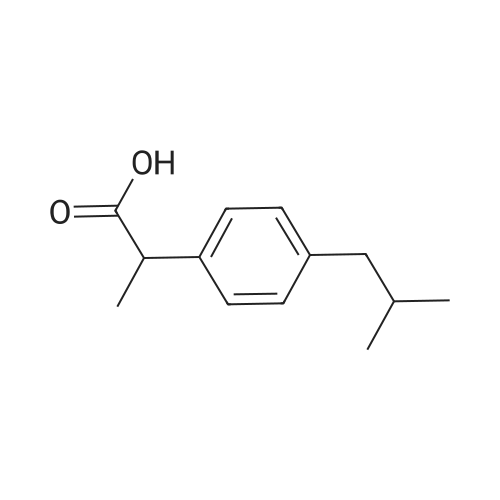
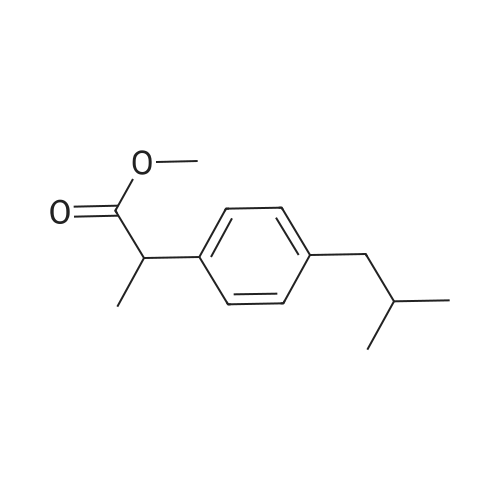
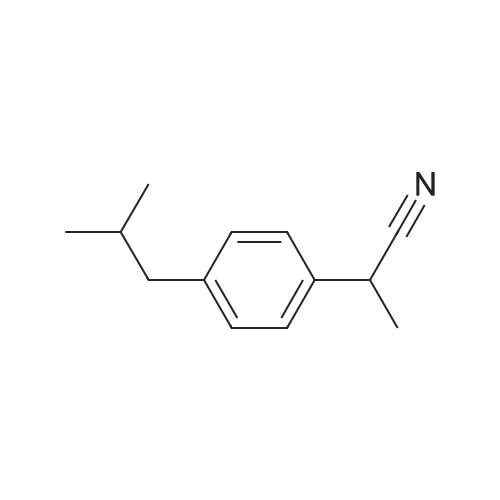
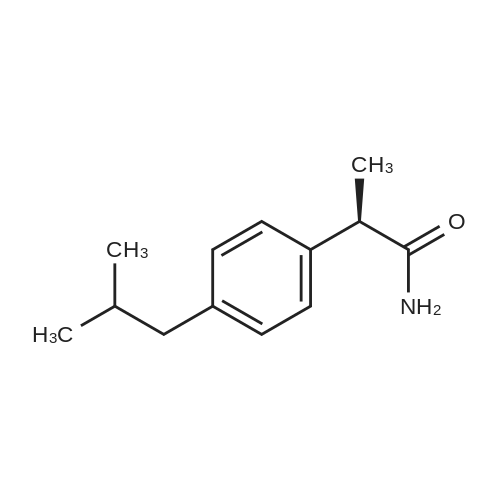
|
With ammonium acetate; In hexane; isopropyl alcohol;Resolution of racemate;Purification / work up; |
Enantiomers of test racemates TR 19-23, of known drugs from the non-steroidal antiinflammatory group, were separated on analytical HPLC columns (250 mm x 4,6 mm), filled with CSP-4 or CSP-3. Since these compounds are structurally free carboxyl acids, it was necessary to use a polar mobile phase containing a certain amount of ammonium acetate. Results obtained by the enantioselective separation of these compounds are shown in Table 5 and the chromatogram achieved for the enantiomers of naproxen is shown in Figure 8. <n="20"/>TABLE 5 Enantiomer separations for test racemate TR 20-23 on the column filled withCSP-3 (250 mm x 4.6 mm ID), with hexane : 2-PrOH = 8:2 + lg/L NH4OAc as the mobile phase, 1 ml/min, 254 nm. |
|
With 2C8H8N5O2(1-)*Cu(2+); In hexane; at 60℃;Resolution of racemate;Catalytic behavior; |
Prior to analysis, the MOFs having repeating units of (SS)-(IA1) of the invention was prepared from the MOFs having repeating units of (SS)-(IBI ') by activation. Particularly, MOFs having repeating units of (SS)-(IBI ') was activated in vacuum at 150C for 12 h on a Schlenk line. 30 mg of the activated MOF having repeating units of (SS)-(IA1) was placed into a solvent as defined in Table 8 at the appropriate temperature as shown in Table 8 with racemic ibuprofen (50% S/R) in a molar ratio (SS)- (IA1):ibuprofen of 1 :3. The reaction was stirred at the temperature indicated in Table 8 overnight. The reaction was filtered to remove the solid, which was washed with a small amount of fresh acetonitrile to remove any ibuprofen sorbed on the surface of the framework. The term "mother liquor" refers to the mixture of the filtrate and the first wash. he amount and chirality of ibuprofen sorbed by the activated MOF was extracted from the solid by suspension in CHCI3 (2 ml) at room temperature for 1 hour. After this time the solid was removed by filtration and the filtrate was analysed by 1H NMR. The enantiomeric excess was determined comparing with the commercially available pure enantiomer, (S)- (+)-lbuprofen or (S)-(+)-4-lsobutyl-a-methylphenylacetic acid, or (S)-(+)-2-(4-lsobutylphenyl)propionic acid (CAS: 51146-56-6, Sigma-Aldrich); and (R)-(+)-lbuprofen or (R)-(+)-4-lsobutyl-a-methylphenylacetic acid or (R)-(+)-2-(4-lsobutylphenyl)propionic acid (CAS: 51146-57-7, Santa Cruz Bio Biotech) using a ultra- performance convergence chromatography (UPPC), ACQUITY UPC2 Waters system with Diode Array Detector; a Column: ChiralPak IA 4.6mm x 100mm, 3mueta, under the following conditions: CO2/ACN/TFA 88:12:0.5, 3ml/min, 1500 psi. Table 8 summarizes the amount of each one of the enantiomer of ibuprofen in the mother liquor and also in the ibuprofen extracted from the MOFs having repeating units of (SS)-(IA1) of the in the invention. Furthermore, Table 8 also specifies the solvent and the temperature of each one of the independent runs: |
|
With colistin sulfate-based polymer monolithic column; In methanol; water; at 20℃;Resolution of racemate; Green chemistry; |
General procedure: The colistin sulfate-based polymer monolithic capillary column was prepared as describedabove and investigated for the nano-LC enantioseparation of a set of different classes of racemicpharmaceuticals, namely: beta-blockers, alpha-blockers, anti-inflammatory drugs, antifungal drugs,norepinephrine-dopamine reuptake inhibitors, catecholamines, sedative hypnotics, antihistamines,antibacterial drugs, anticancer drugs and antiarrhythmic drugs. Although reversed phaseenantio-selective LC examples are limited, macrocyclic antibiotics were previously used inenantioseparation chromatography under reversed phase chromatographic mode [34,36-38,42-45].The initial mobile phase selected for the enantioseparation separation of racemates 1-37 (Figure 3) wasa binary mixture of methanol/water screened from 95:5 to 5:95 nu/nu at 1 mL/min flow rate at fixed UVdetection 219 nm with eleven compounds separated (Rs 1) (Table 1). For examples, in MeOH/H2O80:20 nu/nu, only ibuprofen (7) was separated, while in MeOH/H2O 40:60, indoprofen (10), hexaconazole(15) and miconazole (16) were separated. In MeOH/H2O 10:90 nu/nu, aminoglutethimide (22), tyrosine(29) and O-methoxymandelic acid (34) were also separated. The addition of an additive, namelytriethylamine (TEA) 1% nu/nu in 10:90, resulted in the separation of acebutolol (4) normetanephrine(21), propafenone (26), tyrosine (29) and 4-hydroxy-3-methoxymandelic acid (35) (Figure 4), whilenon-acceptable separations were achieved by addition of the acidic additive namely trifluoroaceticacid (TFA). In an attempt to use normal phase namely n-hexane/2-propanol mixture ranging from10-90% (nu/nu) resulted in resolution less than 1. All chromatographic data are summarized in Table 1. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping