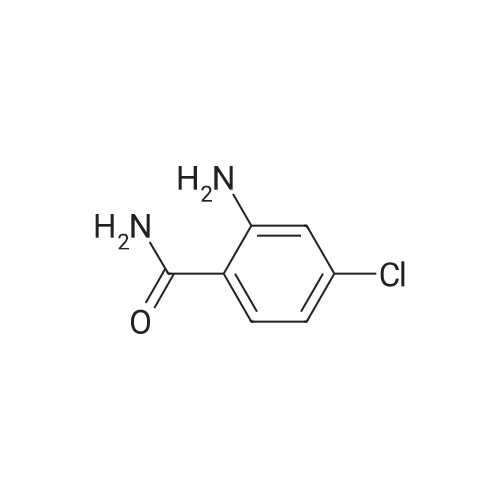
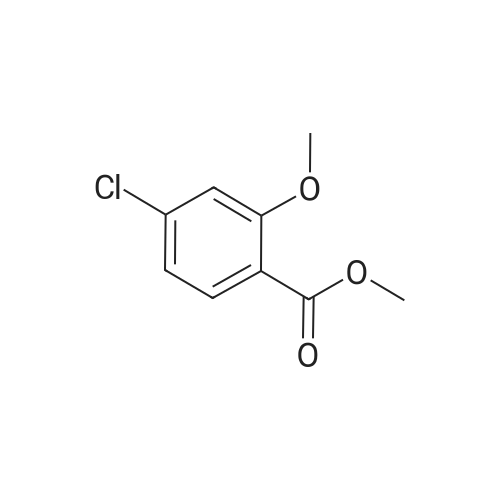
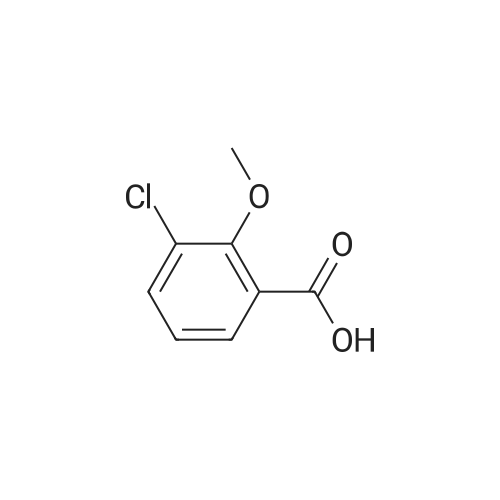
| 83% |
With sulfuric acid; In methanol; ethanol; for 24h;Reflux; |
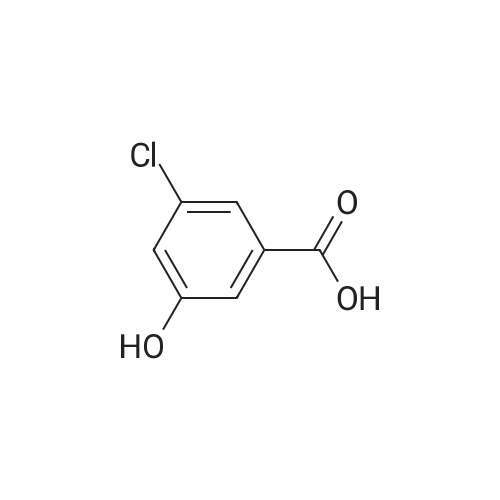
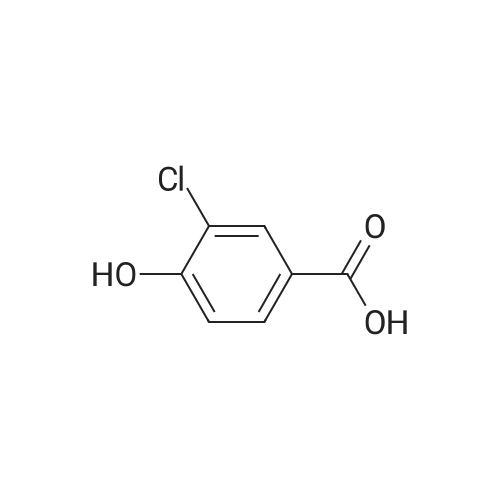
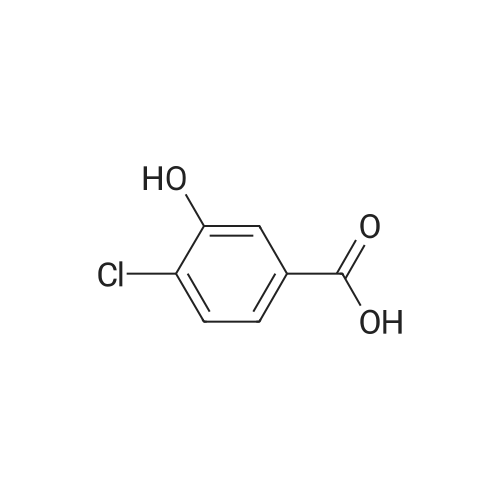
1) Preparation of methyl 4-chloro-salicylate 5 ml of concentrated sulfuric acid is drop-added slowly to 100 ml of methanol. The solution is cooled and then added with a powder of 4-chloro-salicylic acid (17.20g, 0.1 mol), and a reaction is carried out at reflux for 24 hours. After cooling the reaction, a large amount of deposit precipitates and is filtered out, washed with small amount of methanol, and recrystallized in anhydrous ethanol, to obtain 15.60g of methyl 4-chloro-salicylate, with a yield of 83%. |
| 83% |
With sulfuric acid; for 24h;Reflux; |
5 ml of concentrated sulfuric acid was slowly added dropwise to 100 ml of methanol,After cooling the solution, 4-chlorosalicylic acid powder (17.20 g, 0.1 mol) was added, refluxed for 24 hours, cooled and a large amount of precipitate was precipitated, filtered, washed with a small amount of methanol, To give 15.60 g of methyl 4-chlorosalicylate. with a Yield is 83%. |
| 74% |
With sulfuric acid; at 80℃; for 12h; |
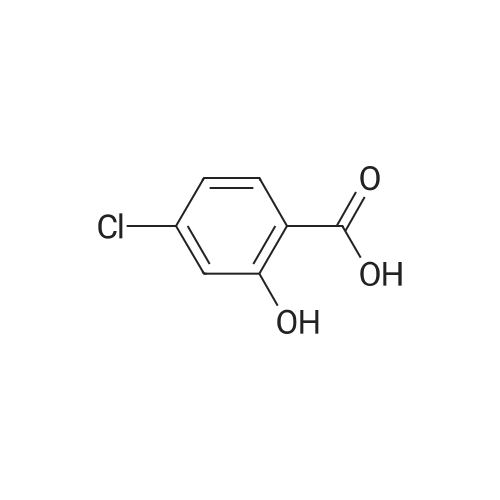
Step 1 Methyl 4-chloro-2-hydroxybenzoate 4-Chloro-2-hydroxybenzoic acid (5.00 g, 28.97 mmol) was dissolved in methanol (50.00 mL), sulfuric acid (4.26 g, 43.46 mmol, 2.32 mL) was added, and then stirred at 80 C. for 12 hours. The reaction was quenched with 30 mL of saturated sodium bicarbonate solution and extracted with dichloromethane (50 mL*3). The organic phases were combined and washed with saturated brine (20 mL), dried over anhydrous sodium sulfate, filtered and concentrated to give methyl 4-chloro-2-hydroxybenzoate (a white solid, 4.0 g, yield: 74.00%). 1H NMR (400 MHz, CDCl3) 10.86 (s, 1H), 7.76 (d, J=8.4 Hz, 1H), 7.00 (s, 1H), 6.88-6.85 (m, 1H), 3.95 (s, 3H). |
| 70% |
With thionyl chloride; at 0℃; for 4h;Inert atmosphere; Reflux; |
SOCl2 (12.6 mL, 174 mmol) was added dropwise into a solution of 240 4-chlorosalicylic acid (10.0 g, 58.0 mmol, TCI reagent) in 27 MeOH (200 mL) at 0C in a nitrogen atmosphere. A resulting mixture was stirred at reflux for 4 hours. After a reaction was completed, a volatile solvent was evaporated under reduced pressure. A saturated NaHCO3 aqueous solution was slowly added into a resulting residue, after which a water layer was extracted with EtOAc. An organic layer was washed with brine, after which the resulting product was dried over anhydrous MgSO4, filtered and concentrated under vacuum. A resulting residue was purified by means of a silica gel column chromatography, so as to obtain the 241 title compound (35-1) (7.6 g, 40.7 mmol, 70%). (0282) 1H NMR (400 MHz, CDCl3); delta 7.76 (d, J = 8.8 Hz, 1H), 7.01 (d, J = 2.0 Hz, 1H), 6.87 (dd, J = 8.4, 2.0 Hz, 1H), 3.95 (s, 3H) |
|
sulfuric acid; In methanol; for 72h;Heating / reflux; |
A solution of 4-chloro-2-hydroxybenzoic acid (3.6 g) and concentrated sulfonic acid (3.6 ml) in methanol (36 ml) was refluxed for 3 days.The resulting mixture was poured into water and the aqueous mixture was extracted with ethyl acetate.The organic layer was washed successively with saturated aqueous sodium bicarbonate and brine, dried over anhydrous magnesium sulfate, evaporated and dried in vacuo to give methyl 4-chloro-2-hydroxybenzoate (3.59 g). NMR (CDCl3, delta): 3.95 (3H, s), 6.87 (1H, dd, J=2.0, 8.5 Hz), 7.01 (1H, d, J=2.0 Hz), 7.76 (1H, d, J=8.5 Hz) |
|
With sulfuric acid; for 72h;Heating / reflux; |
A solution of 4-chloro-2-hydroxybenzoic acid (3.6 g) and concentrated sulfonic acid (3.6 ml) in methanol (36 ml) was refluxed for 3 days. The resulting mixture was poured into water and the aqueous mixture was extracted with ethyl acetate. The organic layer was washed successively with saturated aqueous sodium bicarbonate and brine, dried over anhydrous magnesium sulfate, evaporated and dried in vacuo to give methyl 4-chloro-2-hydroxybenzoate (3.59 g). NMR (CDC13, 5) : 3.95 (3H, s), 6.87 (1H, dd, J=2.0, 8.5Hz), 7.01 (1H, d, J=2. OHZ), 7.76 (1H, d, J=8. 5HZ) |
|
With hydrogenchloride; In water; for 23h;Reflux; |
EXAMPLE 60; [(7-Chloro-4-hydroxy-2-oxo-2H-thiochromene-3-carbonyl)-amino]-acetic acid a) 4-Chloro-2-hydroxy-benzoic acid methyl ester[0330] To a mixture of 4-chlorosalicylic acid (16 g, 92.6 mmol) in MeOH (210 mL) was added cone. HCl solution (5 mL). The resultant solution was refluxed for 23 h. After cooled, solid NaHCO3 was added to neutralize the mixture and then was concentrated. The slurry was suspended in EtOAc and filtered through a silica gel plug, washing with EtOAc. The filtrate was washed with 1A saturated NaHCO3 solution (2x), brine, dried over Na2SO4, filtered and concentrated to provide the title compound (17.2 g). MS ESI(-) m/e: 185.0 (M-I). |
|
With sulfuric acid; at 80℃; |
General procedure: To a solution of 1a (5 mmol) in CH3OH (25 mL) concentrated sulfuric acid was added dropwise (10 drops). After stirring under reflux for 12 h, the reaction mixture was concentrated under reduced pressure, diluted with water, and extracted with ethyl acetate. The organic layer was washed with saturated aqueous NaHCO3 solution and brine, dried over Na2SO4, and concentrated under reduced pressure. The crude mixture was purified by flash chromatography (petroleum ether/ethyl acetate = 5:1) to afford 2a. |
|
With thionyl chloride; at 0 - 65℃; for 48.16h; |
General procedure: Methyl 4-bromosalicylate (1 mol) was dissolved in methanol(25 mL), and thionyl chloride was added dropwise under ice-cooling,after 10 min, The mixture was stirred at 65 C for 48 h. and monitoredby TLC. After the reaction was completed, excess methanol was evaporated,the residue was extracted with ethyl acetate and aqueous sodiumcarbonate, and the organic phase was added to anhydrous sodiumsulfate and concentrated to obtain intermediate 6a.The compounds 6b-6h were prepared analogously. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping