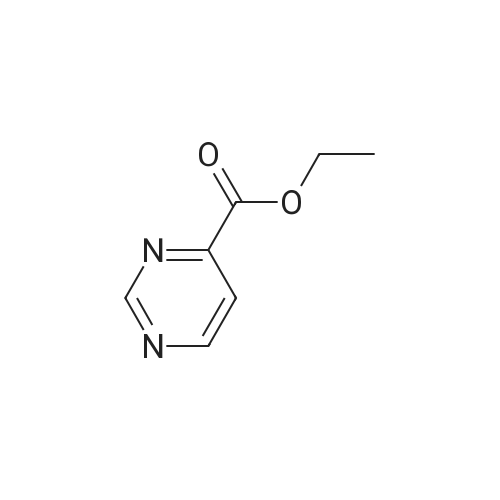
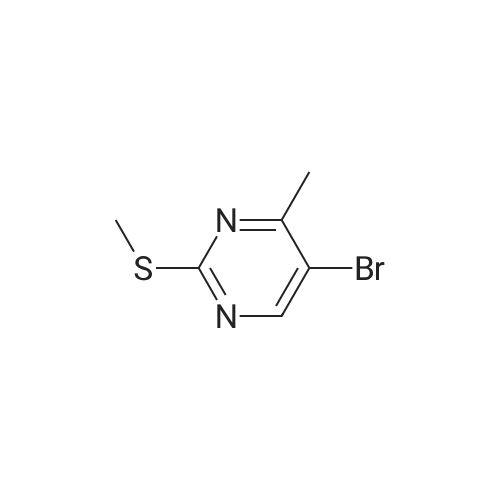
| 10% |
With sodium acetate; In N,N-dimethyl-formamide; at 120℃; for 0.666667h;Microwave; |
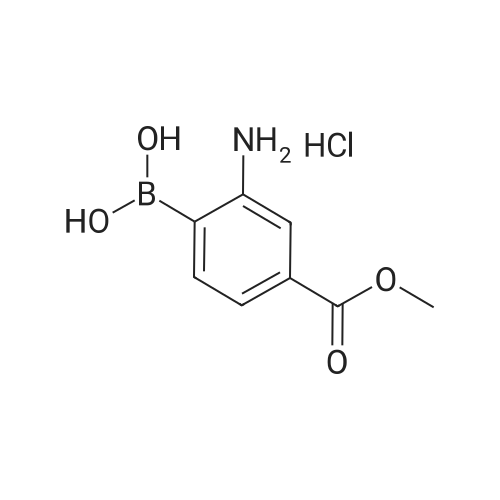
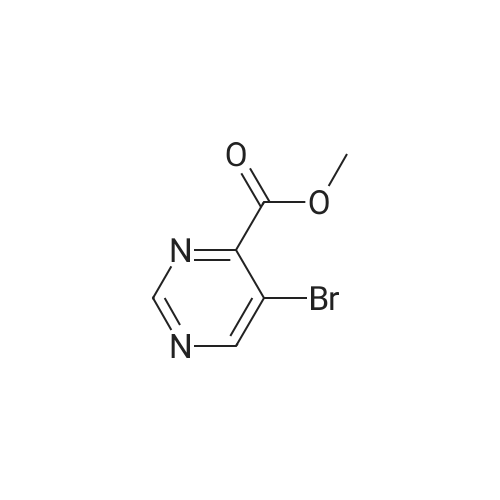
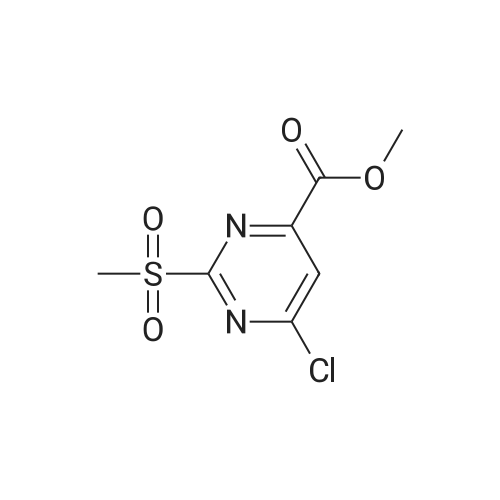
In a microwave vessel, methyl 5-bromo-2-(methylthio)pyrimidine-4- carboxylate (1.0 eq, 274 mg, 1.18 mmol), <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.2 eq, 329 mg, 1.42 mmol), and sodium acetate (3.0 eq, 291 mg, 3.55 mmol) were mixed in anhydrous DMF (2 ml). The mixture was degassed by bubbling nitrogen gas in the solution for 10 min and the reaction heated under microwaves at 1200C for 30 min. After cooling down the expected material crashed out of NMP. The solid was filtered, suspended in water filtered and dried. The material was triturated in AcOEt and filtered give a yellow solid. The same procedure was repeated 9 times using the same amounts of materials to provide methyl 3-(methylthio)-5-oxo-5,6-dihydropyrimido[4,5-c]quinoline-8- carboxylate (283 mg, 10% yield). LCMS (ES): >95% pure, m/z 302 [M+l]+, 1H NMR <n="82"/>(DMSO-d6, 400 MHz) delta 2.71 (s, 3H), 3.89 (s, 3H), 7.80 (dd, / = 1.6, / = 8.4, IH), 7.97 (d, / = 1.6, IH), 8.59 (d, / = 8.8, IH), 9.98 (s, IH), 12.34 (s, IH) ppm. |
| 10% |
With sodium acetate; In N,N-dimethyl-formamide; at 120℃; for 0.666667h;Microwave irradiation; |
Process 8 In a microwave vessel, methyl 5-bromo-2-(methylthio)pyrimidine-4-carboxylate (1.0 eq, 274 mg, 1.18 mmol), <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.2 eq, 329 mg, 1.42 mmol), and sodium acetate (3.0 eq, 291 mg, 3.55 mmol) were mixed in anhydrous DMF (2 ml). The mixture was degassed by bubbling nitrogen gas in the solution for 10 min and the reaction heated under microwaves at 120 C. for 30 min. After cooling down the expected material crashed out of NMP. The solid was filtered, suspended in water filtered and dried. The material was triturated in AcOEt and filtered give a yellow solid. The same procedure was repeated 9 times using the same amounts of materials to provide methyl 3-(methylthio)-5-oxo-5,6-dihydropyrimido[4,5-c]quinoline-8-carboxylate (283 mg, 10% yield). LCMS (ES): >95% pure, m/z 302 [M+l]+, 1H NMR (DMSO-d6, 400 MHz) delta 2.71 (s, 3H), 3.89 (s, 3H), 7.80 (dd, J=1.6, J=8.4, 1H), 7.97 (d, J=1.6, 1H), 8.59 (d, J=8.8, 1H), 9.98 (s, 1H), 12.34 (s, 1H) ppm. |
| 10% |
With sodium acetate; In N,N-dimethyl-formamide; at 120℃; for 0.666667h;Microwave irradiation; |
In a microwave vessel, methyl 5-bromo-2-(methylthio)pyrimidine-4-carboxylate (1.0 eq, 274 mg, 1.18 mmol), <strong>[380430-55-7]2-amino-4-(methoxycarbonyl)phenylboronic acid hydrochloride</strong> (1.2 eq, 329 mg, 1.42 mmol), and sodium acetate (3.0 eq, 291 mg, 3.55 mmol) were mixed in anhydrous DMF (2 ml). The mixture was degassed by bubbling nitrogen gas in the solution for 10 min and the reaction heated under microwaves at 120 C. for 30 min. After cooling down the expected material crashed out of NMP. The solid was filtered, suspended in water filtered and dried. The material was triturated in AcOEt and filtered give a yellow solid. The same procedure was repeated 9 times using the same amounts of materials to provide methyl 3-(methylthio)-5-oxo-5,6-dihydropyrimido[4,5-c]quinoline-8-carboxylate (283 mg, 10% yield). LCMS (ES): >95% pure, m/z 302 [M+1]+, 1H NMR (DMSO-d6, 400 MHz) delta 2.71 (s, 3H), 3.89 (s, 3H), 7.80 (dd, J=1.6, J=8.4, 1H), 7.97 (d, J=1.6, 1H), 8.59 (d, J=8.8, 1H), 9.98 (s, 1H), 12.34 (s, 1H) ppm. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +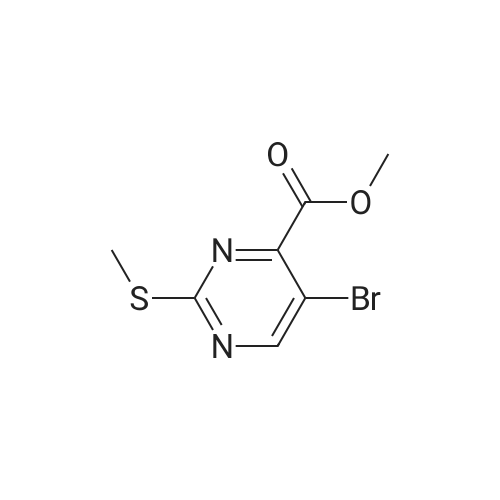

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping