| 76% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In 1,4-dioxane; at 20 - 105℃; for 13.1667h;Inert atmosphere; |
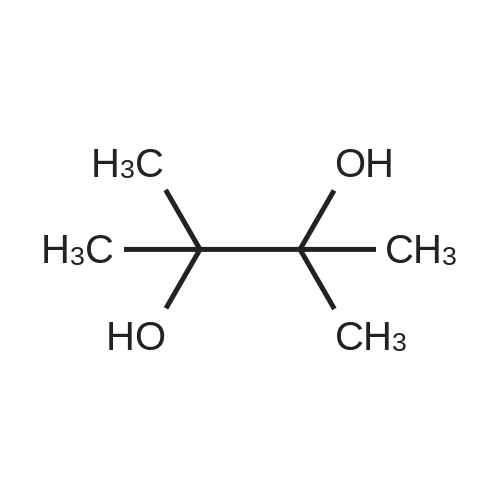
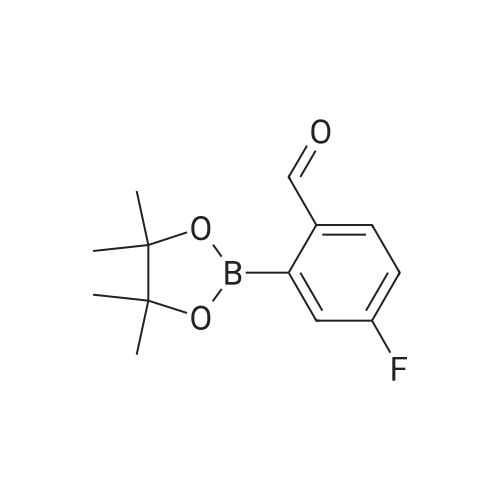
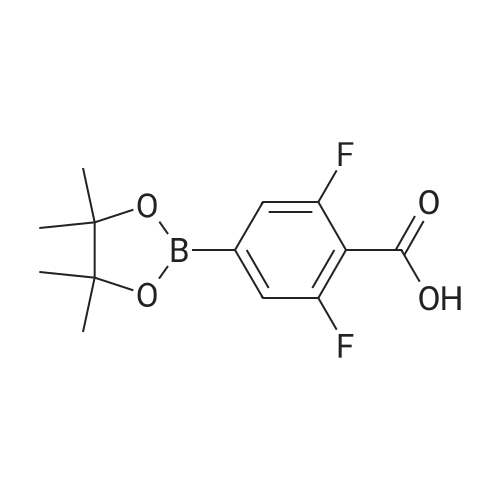
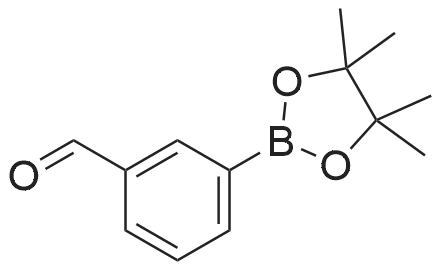
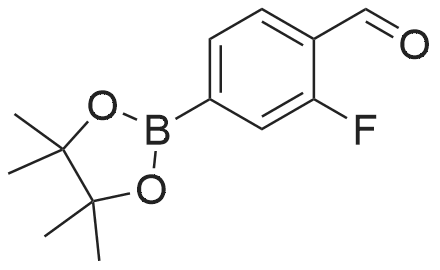
(S)-2-fluoro-4-(2-(2-((6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl)morpholino)pyrimidin-5-yl)benzaldehyde In a pressure tube reactor, (S)-4-(5-bromopyrimidin-2-yl)-2-((6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)methyl)morpholine (50 mg, 0.11 mmol) was added, and then 1M Na2CO3 (0.33 mL, 0.33 mmol) was added. Pd(PPh3)4 (6 mg, 0.005 mmol) was further added, and then 1,4-dioxane (1 mL) and <strong>[503176-50-9]2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2-yl)benzaldehyde</strong> (41 mg, 0.16 mmol) were added. The mixture was stirred at room temperature for 10 minutes under nitrogen gas, and then stirred at 105 C. for 13 hours. When the reaction was completed, the organic layer was extracted with ethyl acetate and water, and the extra water was removed by sodium sulfate, followed by concentration under reduced pressure. Solid was generated using ether and hexane, followed by filtration, to give (S)-2-fluoro-4-(2-(2-((6-(1-methyl-1H-pyrazol-4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-1-yl)-methyl)morpholino)pyrimidin-5-yl)benzaldehyde (41 mg, 0.087 mmol) with a yield of 76%. 1H-NMR (300 MHz, CDCl3) delta 10.36 (s, 1H), 8.92 (s, 1H), 8.59 (s, 2H), 8.17 (s, 1H), 8.12 (s, 1H), 7.94 (t, J=7.56 Hz, 1H), 7.36 (d, J=8.1 Hz, 1H), 4.99-4.92 (m, 1H), 4.85-4.79 (m, 1H), 4.54 (d, J=12.9 Hz, 1H), 4.27 (brs, 1H), 4.02 (s, 3H), 3.64-3.56 (m, 1H), 3.28-3.13 (m, 2H) |
| 76% |
With tetrakis(triphenylphosphine) palladium(0); sodium carbonate; In 1,4-dioxane; at 20 - 105℃; for 13.1h;Inert atmosphere; |
[0295] In a pressure tube reactor (S) -4- (5- bromopyrimidin-2-yl) -2 - ((6- (1-methyl -1H- pyrazol-4-yl) -1H- [1,2,3] triazole pyrazolo [4,5- b] pyrazin-1-yl) methyl) morpholine (50 mg, 0.11 mmol) was added, followed by the addition of 1 M Na2CO3 (0.33 mL, 0.33 mmol) . Pd (PPh3) 4 (6 mg, 0.005 mmol) was further added, and 1,4-dioxane (1 mL) and 2-fluoro-4- (4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzaldehyde (41 mg, 0.16 mmol), and the mixture was stirred at room temperature under nitrogen gas for 10 minutes and then at 105 DEG C for 13 hours. After the reaction was completed, the organic layer was extracted with ethyl acetate and water, and the excess water was removed with anhydrous magnesium sulfate and concentrated under reduced pressure. (S) -2-fluoro-4- (2- (2 - ((6- (1 -methyl-1 H-pyrazol-4-yl) -1H Yl) benzaldehyde (41 mg, 0.087 mmol) was obtained in 76% yield according to a procedure similar to that used for the synthesis of . |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping