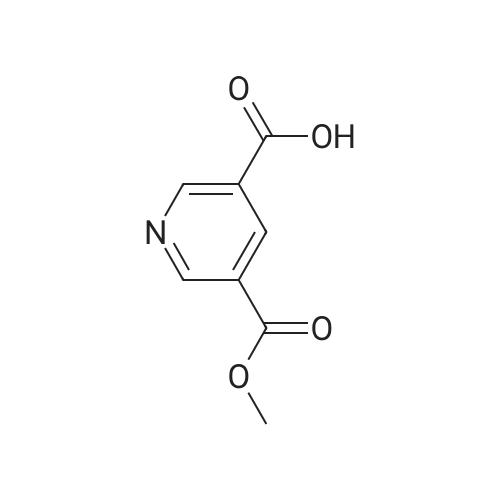
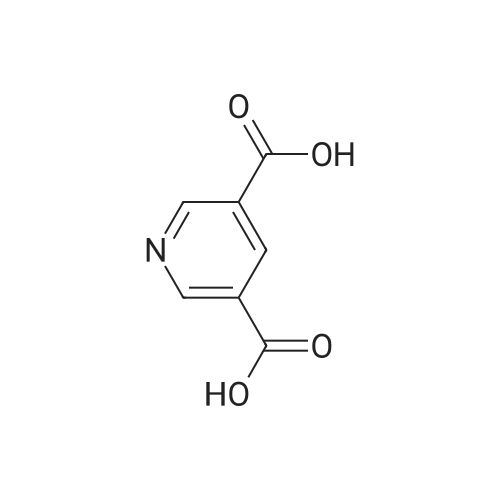
|
With sulfuric acid; In methanol; at 120℃; for 2h;Microwave irradiation; |
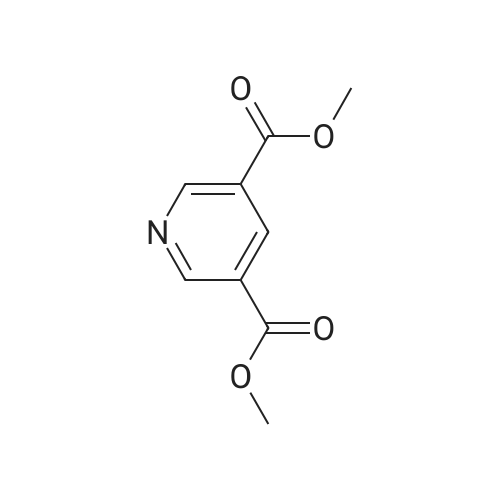
A: Pyridine-3,5-dicarboxylic acid dimethyl ester 3,5-Pyridinedicarboxylic acid (1.5 g, 63 mmol) and conc. H2SO4 (0.9 mL) in MeOH (15 mL) are heated in a microwave oven at 120 C. for 2 h. The solvent is evaporated to give a residue which is partitioned between ethyl acetate and sat. aq. NaHCO3. The organic phase is washed with brine, dried over Na2SO4, filtered and evaporated to give a light yellow solid. MS (LC-MS): 196 [M+H]+ TLC, Rf (ethyl acetate/hexane 1:1)=0.56. |
|
|
3,5-Pyridinedicarboxylic acid (1.5 g, 63 mmol) and conc. H2SO4 (0.9 mL) in MeOH (15 mL) are heated in a microwave oven at 120 C. for 2 h. The solvent is evaporated to give a residue with is partitioned between ethyl acetate and sat. aq. NaHCO3. The organic phase is washed with brine, dried over Na2SO4, filtered and evaporated to give a light yellow solid. MS (LC-MS): 196 [M+H]+ TLC, Rf (ethyl acetate/hexane 1:1)=0.56. |
|
With thionyl chloride; at 10 - 35℃;Reflux; |
Reference Example 1dimethyl pyridine-3,5-dicarboxylate Pyridine-3,5-dicarboxylic acid (25.5 g) was suspended in methanol (184 ml), and thionyl chloride (33.8 ml) was added dropwise at room temperature. The reaction mixture was stirred with heating under reflux for 3 hr, and the mixture was allowed to cool to room temperature and concentrated under reduced pressure. The residue was diluted with water, and the mixture was extracted with ethyl acetate. The aqueous layer was neutralized with 8M aqueous sodium hydroxide solution, and extracted with ethyl acetate. The extract was washed successively with saturated aqueous sodium hydrogen carbonate solution and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give the object product (27.9 g) as a powder.1H-NMR (CDCl3) delta 4.00 (6H, s), 8.88(1H, t), 9.37(2H, d) |
|
|
Reference Example 33 dimethyl pyridine-3,5-dicarboxylate [Show Image] Pyridine-3,5-dicarboxylic acid (100 g) was suspended in methanol (1000 ml), and thionyl chloride (130 ml) was added dropwise at room temperature. The reaction mixture was stirred with heating to reflux for 3 hr. The mixture was allowed to cool to room temperature, and concentrated under reduced pressure. The residue was diluted with water, and the mixture was extracted with ethyl acetate. The aqueous layer was neutralized with 8 M aqueous sodium hydroxide solution, and the mixture was extracted with ethyl acetate. The extract was washed successively with saturated aqueous sodium hydrogen carbonate solution and saturated brine, and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give the object compound (117 g) as a powder. 1H-NMR (CDCl3) delta 4.00 (6H, s), 8.88 (1H, t), 9.37 (2H, d). |
|
With thionyl chloride; at 60℃; for 12h; |
Dimethyl pyridine-3,5-dicarboxylateTo a solution of pyridine-3,5-dicarboxylic acid (85.0 g, 508.62 mmol) in MeOH (1 L) was added SOCl2 (61.80 g, 519.46 mmol) dropwise. The reaction mixture was stirred and heated to 60 C for 12 hr. The reaction mixture was concentrated under vacuum to afford the title compound (120.00 g, crude) as a white solid. |
|
|
3,5-Pyridinedicarboxylic acid (1.5g, 63 mmol) and cone. H2SO4 (0.9 mL) in MeOH (15 mL) are heated in a microwave oven at 120C for 2 h. The solvent is evaporated to give a residue with is partitioned between ethyl acetate and sat. aq. NaHCO3. The organic phase is washed with brine, dried over Na2SO4, filtered and evaporated to give a light yellow solid. MS (LC-MS): 196 [M+H]+ TLC, Rf (ethyl acetate/hexane 1 :1 ) = 0.56. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping