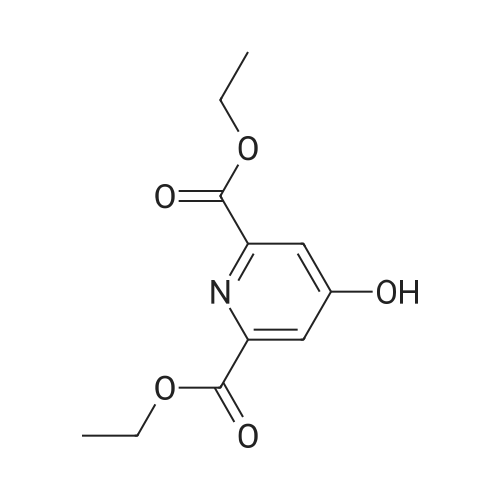
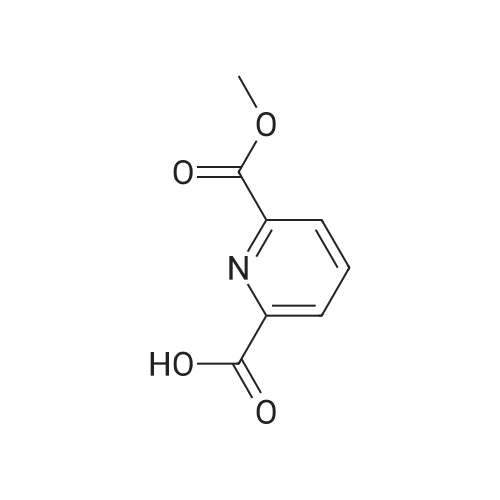
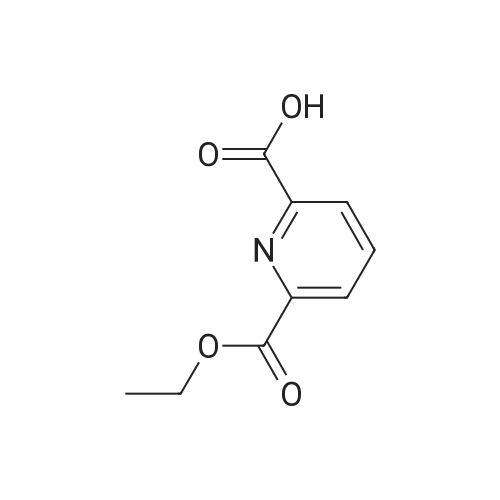
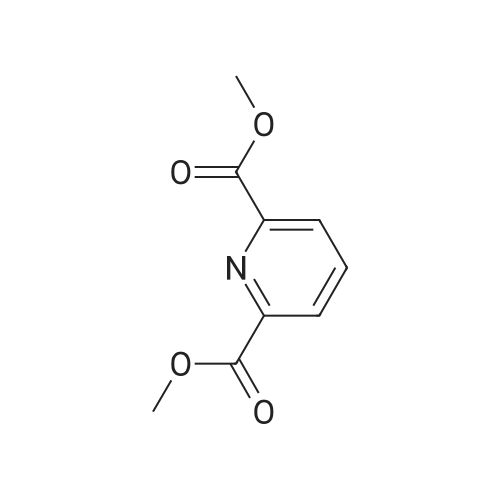
| 63% |
With sulfuric acid; In water; for 16h;Reflux; |
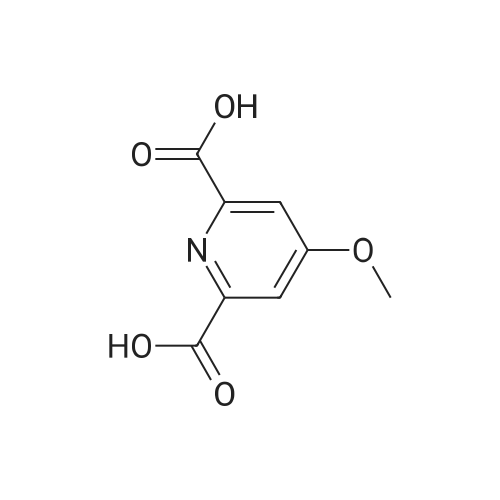
The compound 37 was prepared according to the procedure described in the article Chemistry-A European Journal 2008, 14, 1726. The chelidamic acid monohydrate 5 (3 g, 15 mmol) was heated at reflux in EtOH (60 ml) in the presence of 97% sulfuric acid (0.6 ml) for 16 h. The solvent was evaporated under reduced pressure, the white residue remaining was neutralized with a saturated aqueous solution of sodium bicarbonate and extracted with DCM. The organic phase was dried with magnesium sulfate, evaporated to dryness and the diester 37 was obtained in the form of a white solid (2.27 g, 63%). Rf=0.10 (silica, CH2Cl2/MeOH 99/1); 1H NMR (CDCl3, 300 MHz) delta 7.31 (s, 2H), 4.46 (q, J=7.1 Hz, 4H), 1.42 (t, J=7.1 Hz, 6H); 13C NMR (DMSO-d6, 62.5 MHz) delta 166.0, 164.3, 149.9, 115.2, 61.4, 14.1. |
| 56% |
With thionyl chloride; at 0℃;Heating / reflux; |
Example 35; 411 412 413Part A To chelidamic acid monohydrate 403 (12.89 g, 64.1 mmol) in ethanol (100 ml) in an ice bath was added thionyl chloride (5 ml_) slowly. The ice bath was removed and the reaction mixture was heated to reflux overnight. The ethanol was removed in vacuo and the residue was dissolved in ethyl acetate (50 ml_). The mixture was washed with saturated sodium bicarbonate until the aqueous layer was no longer acidic. The organic layer was washed with saturated sodium chloride solution, dried over sodium sulfate and concentrated to afford a tacky white solid (11.64 g). Recrystallization of the white solid (9.25 g) from ethyl acetate/hexane/DCM afforded a crystalline white solid (7.44 g, 56%) 1H NMR (300 MHz, CDCI3) delta 7.31 (bs, 2H), 4.43 (q, 4H, J = 7.1 Hz), 1.40 (t, 6H, J = 7.1 Hz). |
|
With toluene-4-sulfonic acid;Industry scale; Reflux; |
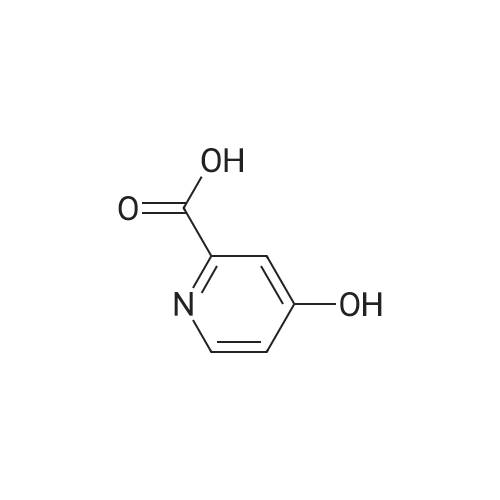
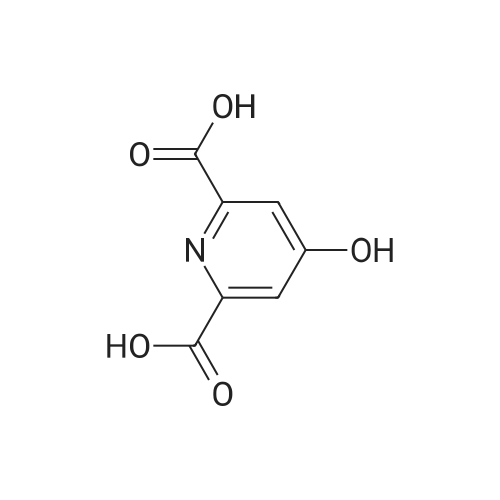
Reference Example 2-1; 4-hydroxypyridin-2.6-dicarboxylic acid diethyl esterIn ethanol (60.0 L) was dissolved 4-hydroxypyridin-2,6-carboxylic acid hydrate (3.00 kg). To the solution was added p-toluenesulfonic acid monohydrate (514 g), and the mixture was heated to reflux overnight. The reaction solution was concentrated under reduced pressure, a saturated aqueous sodium hydrogencarbonate solution was added to the residue, and the mixture was extracted with chloroform. The organic layer was washed with a saturated saline solution and dried over anhydrous sodium sulfate. Insoluble matters were filtered out, and the filtrate was concentrated under reduced pressure to give the title compound (3.48 kg) as a yellow oil. mass :240(M+ 1)+ . |
|
With toluene-4-sulfonic acid; In water;Reflux; |
Reference Example 2-1 4-hydroxy-2,6-pyridinedicarboxylic acid diethyl ester In ethanol (60.0 L) was dissolved 4-hydroxy-2,6-pyridinecarboxylic acid hydrate (3.00 kg). To the solution was added p-toluenesulfonic acid monohydrate (514 g), and the mixture was heated to reflux overnight. The reaction solution was under reduced pressure, a saturated aqueous sodium hydrogencarbonate solution was added to the residue, and the mixture was extracted with chloroform. The organic layer was washed with a saturated saline solution and dried over anhydrous sodium sulfate. Insoluble matters were filtered out, and the filtrate was under reduced pressure to give the title compound (3.48 kg) as a yellow oil. mass: 240 (M+1)+. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping