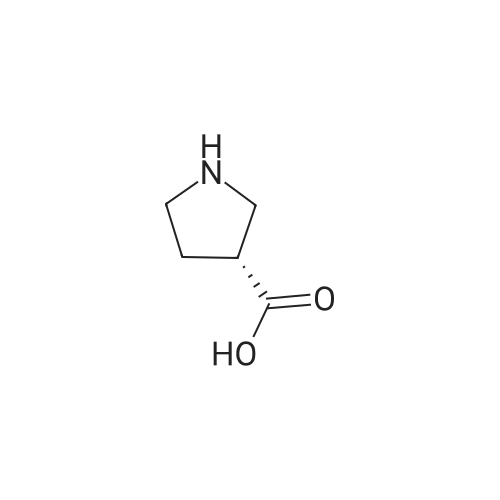
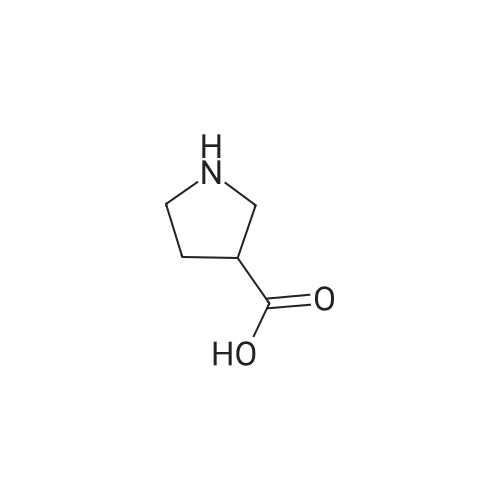
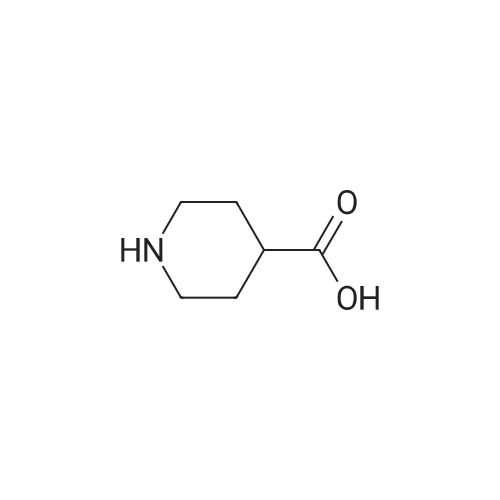
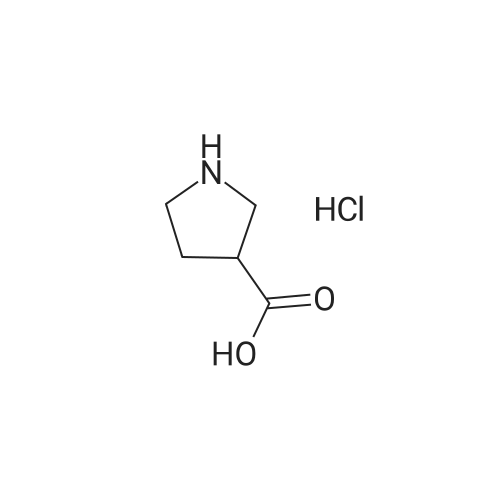
|
|
[1246] To a solution of nipecotic acid (10 g, 63.6 mmol) in 1 N NaOH (2.5 g in 64 mL water, 63.6 mmol) at 0 C. was alternately added benzyloxycarbonyl chloride (10.9 mL, 76.5 mmol) in diethyl ether (50 mL) and 1 N NaOH (5 g in 128 mL water, 127.2 mmol) in five portions. The reaction mixture was stirred at 0 C. for 2 h, and at ambient temperature for 24 h. Then this was made acidic with 10% HCl and the solid formed was filtered and dried (vacuum oven, 45 C.) to obtain the title compound (18.9 g, 113%). MS (ESI) m/e 264 (M+H)+. |
|
|
EXAMPLE 15 ()-BENZYL 3- (6-METHOXY-2-METHYL-1-OXO-4-PHENYL-1, 2-DIHYDROISOQUINOLIN-3-YL) PIPERIDINE-1- carboxylate; Step A: ()-L-F (BENZYLOXY) CARBONYLLPIPERIDINE-3-CARBOXYLIC ACID; To an ice cooled solution of nipecotic acid (10.0 g, 77.5 mmol), sodium hydroxide (3.4 g, 85 mmol), and tetrahydrofuran (50 ML) in water (100 ML) was added by simultaneous dropwise addition benzylchloroformate (13.3 ML, 93 mmol) in tetrahydrofuran (50 ML) and sodium hydroxide (3.4 g, 85 mmol) in water (50 mL). Warmed slowly to room temperature. After 24 hours tetrahydrofuran was removed in vacuo and the resulting aqueous mixture acidified with 3 N hydrochloric acid and extracted with dichloromethane (3X). The combined organic portions were dried with anhydrous magnesium sulfate. Filtration followed by evaporation of the filtrate in vacuo gave the titled COMPOUND. HNMR (CHCIS, 300MHZ) Q 7.45-7. 20 (m, 5H) ; 5.14 (m, 2H); 4.21 (br S, 1H) ; 3.96 (m, 1H), 3.15 (br s, 1H) ; 2.93 (m, 1H) ; 2.51 (m, 1H) ; 2.09 (m, 1H); 1.80-1. 60 (m, 2H); 1.50 (m, 1H) ppm |
|
|
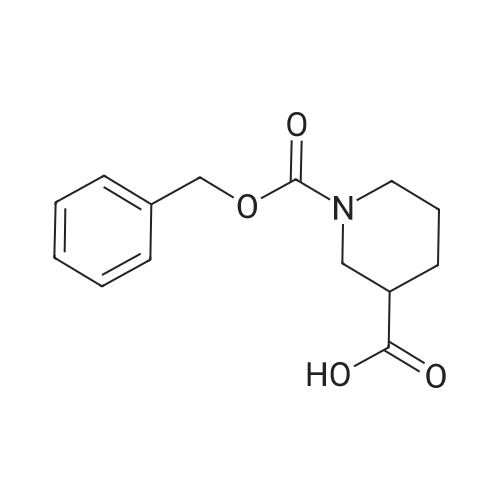
To a stirred solution of 3-piperidine carboxylic acid (1.48 g, 11.5 mmol) and saturated sodium bicarbonate (40 mL) in tetrahydrofuran (40 mL) at 0 C. was added benzylchloroformate (2.05 g, 12.0 mmol). The mixture was stirred in an ice-bath for 3 h, and then at room temperature for 16 h. The mixture was then cooled to 0 C. and the pH was reduced to ca. 1.0 with 6 M HCl. The mixture was extracted three times with ethyl acetate. The combined extracts were dried with magnesium sulfate, filtered, and concentrated to provide 10.0 g of the title compound as a colorless oil. |
|
|
Step 1. 1-(Benzyloxycarbonyl)piperidine-3-carboxylic; A 500-mL 4-necked round-bottom flask was charged with a solution of NaOH (8 g, 198.00 mmol, 1.00 equiv, 99%) in H2O (200 mL). To this was added piperidine-3-carboxylic acid (25.8 g, 197.75 mmol, 1.00 equiv, 99%), in small portions at 0 C. Then, a solution of benzyl carbonochloridate (39.2 g, 227.48 mmol, 1.15 equiv, 99%) in Et2O (50 mL) was added at 0 C. over 40 minutes. Then a solution of NaOH (12 g, 1.50 equiv) in H2O (300 mL) was added drop wise with stirring at 0-10 C. The resulting solution was allowed to stir overnight at room temperature. The reaction progress was monitored by TLC (EtOAc/PE=1/1). The pH adjusted to 3 with 10% aqueous HCl. The resulting solution was extracted with ethyl acetate (3×500 mL). Combined organic layers were dried over anhydrous magnesium sulfate and concentrated on a rotary evaporator to afford 1-(benzyloxycarbonyl)piperidine-3-carboxylic acid (58 g) as white solid. |
|
With sodium hydroxide; In water; at 0 - 20℃; |
Step 1: In a 100 ml three-necked flask, in a stream of nitrogen and under magnetic stirring the nipecotinic acid (3.7 g) was placed in solution in a 3N sodium hydroxide solution (30 ml). The solution was cooled over an ice bath to 0 C. Benzyl chloroformiate (5.8 ml) was added in fractions at 0 C. alternately with a 3N sodium hydroxide solution (9.3 ml) for a time of 45 min. After the addition time the reaction medium was pale yellow and the temperature 3 C. The ice bath was removed and the reaction medium left under stirring at ambient temperature overnight. The aqueous phase was then extracted once with ethyl ether, then acidified to pH 1 using a 3N hydrochloric acid solution. The acidified aqueous phase was extracted three times with ethyl ether. The ether extract phase was successively washed with 1N hydrochloric acid solution, then twice with saturated sodium chloride solution before being dried over magnesium sulphate, filtered then concentrated in vacuo. [0126] The yellow oil obtained (7.35 g, 98%) was used at the following step without any purification step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping