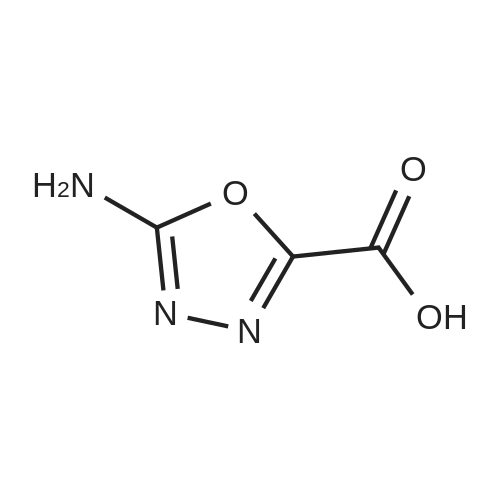
Alternatived Products of [ 4970-53-0 ]
Product Details of [ 4970-53-0 ]
| CAS No. : | 4970-53-0 |
MDL No. : | MFCD03425200 |
| Formula : |
C5H7N3O3
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | JZSBFOOEPYPCPW-UHFFFAOYSA-N |
| M.W : |
157.13
|
Pubchem ID : | 2756523 |
| Synonyms : |
|
Application In Synthesis of [ 4970-53-0 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 4970-53-0 ]
- 1
-
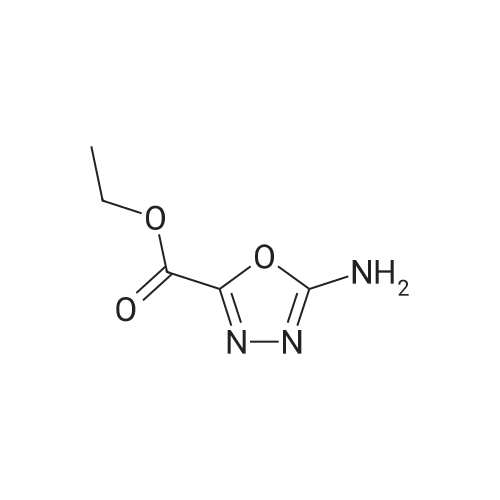 [ 4970-53-0 ]
[ 4970-53-0 ]

-
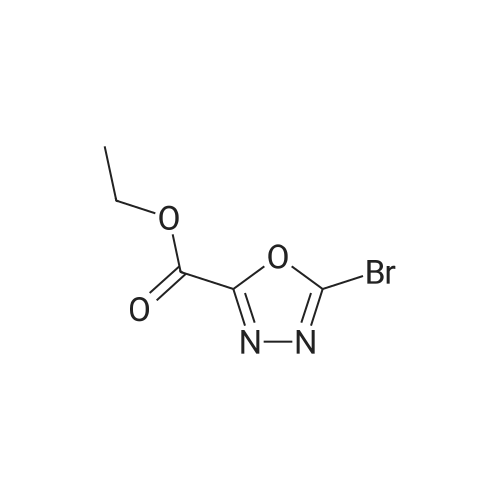 [ 916889-45-7 ]
[ 916889-45-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
EXAMPLE 19; 5-[(S)-3-(2-Bromo-5-fluoro-phenoxy)-pyrrolidin-l-yll-ri,3,41oxadiazole-2-carboxamide; Step 1 : Ethyl 5-bromo-l ,3,4-oxadiazole-2-carboxylate; To a suspension of ethyl 5-amino-l,3,4-oxadiazole-2-carboxylate (8.8 g, 56 mmol) in CH3CN (187 mL) was added CuBr2 (18.8g, 84 mmol). The mixture turned dark green <n="49"/>and further stirred for 15 min at room temperature. t-BuONO, 90% (15 mL, 112 mmol) was added and stirred at room temperature for 2h then heated at 50 0C for 0.5h. The solvent was then evaporated in vacuo. Water (100 mL) and EtOAc (100 mL) were added and the mixture was filtered through celite and washed with EtOAc. The EtOAc layer was separated, and the aqueous layer extracted with EtOAc (2 x 50 mL). The combined organic layers were dried over Na2SO4 and concentrated. The product was recrystallized from CH2Cl2/hexanes to give the title compound as solid. 1H NMR (400 MHz, CDCl3): delta 4.56 (q, 2H), 1.51 (t, 3H). |
- 2
-
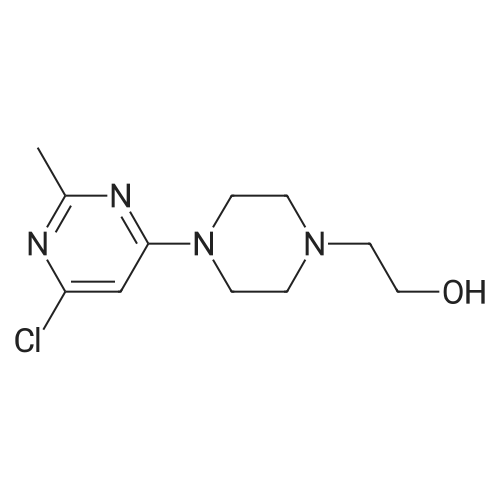 [ 127116-19-2 ]
[ 127116-19-2 ]

-
 [ 4970-53-0 ]
[ 4970-53-0 ]

-
5-{6-[4-(2-hydroxy-ethyl)piperazin-1-yl]-2-methylpyrimidin-4-ylamino}-[1,3,4]oxadiazole-2-carboxylic acid ethyl ester
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
| 20% |
With palladium diacetate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; sodium t-butanolate; In N,N-dimethyl-formamide; at 110℃; for 36h;Schlenk technique; Inert atmosphere; |
General procedure: A 10-ml Schlenk tube equipped with a stir-bar was charged with compound 1 (0.2 mmol), compound 2 (0.3 mmol), Pd(OAc)2 (0.02 mmol), Xantphos (0.04 mmol), NaOtBu (0.3 mmol), anhydrous DMF (2 mL).The reaction tube was purged with argon. The Schlenk tube was placed in an oil-bath at 110 oC for 36 hours and then cooled to room temperature. The reaction mixture was extracted with EtOAc (3 × 20 mL). The combined organic phase was dried over MgSO4 and concentrated under reduced pressure and the crude residue purified by flash chromatography on silica gel. The purified material was dried in vacuo to afford the corresponding products 3a-w. |
| 20% |
With xantphos; palladium diacetate; sodium t-butanolate; In N,N-dimethyl-formamide; at 110℃; for 36h;Schlenk technique; Inert atmosphere; |
In a 10 ml Schlenk tube, add compound 1c (51.2 mg, 0.2 mmol), 5-amino-[1,3,4]oxadiazole-2-carboxylic acid ethyl ester (47.1 mg, 0.3 mmol), Pd (OAc)2 (4.5 mg, 0.02 mmol), xantphos (23 mg, 0.04 mmol), NaOtBu (29 mg, 0.3 mmol) and anhydrous DMF (2 mL).Under the protection of argon, the reaction tube was placed in an oil bath at 110 C. for 36 h for Buchwald-Hartwig reaction, and then cooled to room temperature. The reaction mixture was extracted with EtOAc (3 × 20 mL). The organic phase was dried over MgSO4 and concentrated under reduced pressure. The corresponding product 2e (15 mg) was separated on a silica gel column, white solid, melting point: 134 C, yield 20%, purity 96%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping