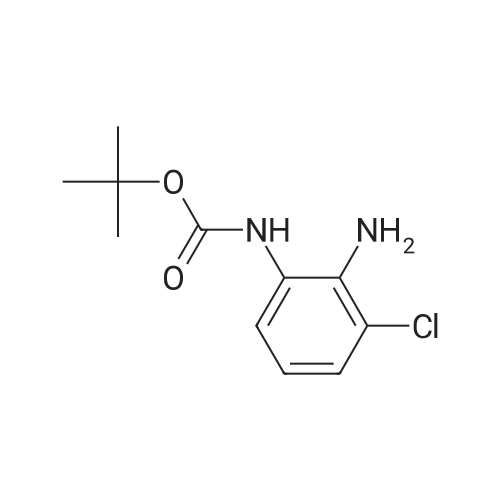
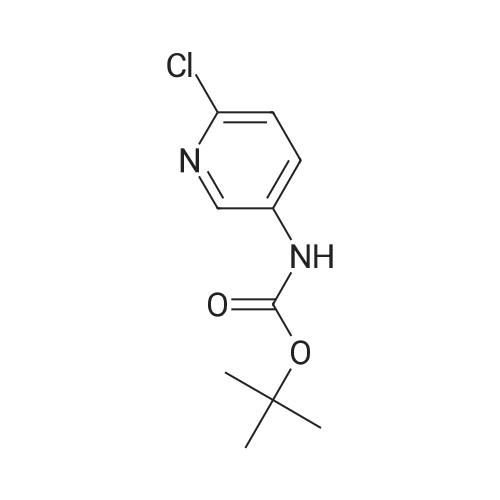
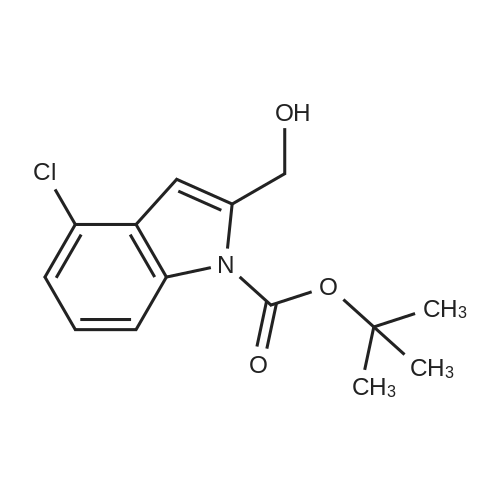
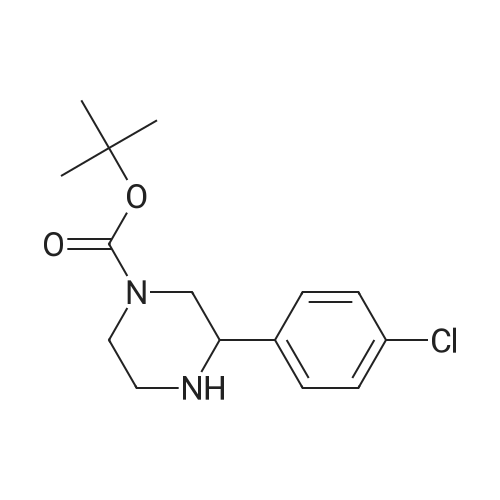
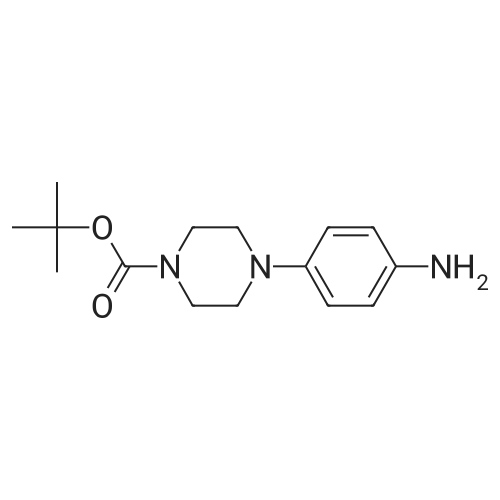
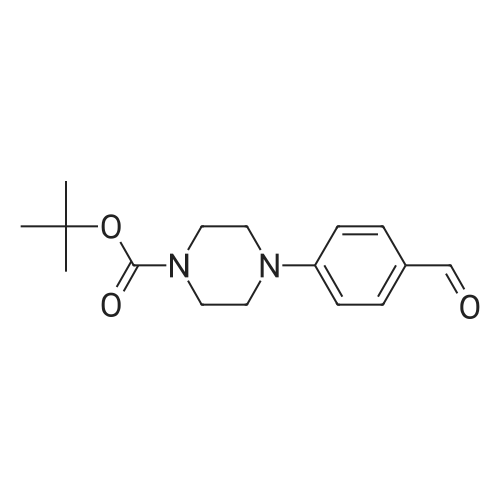
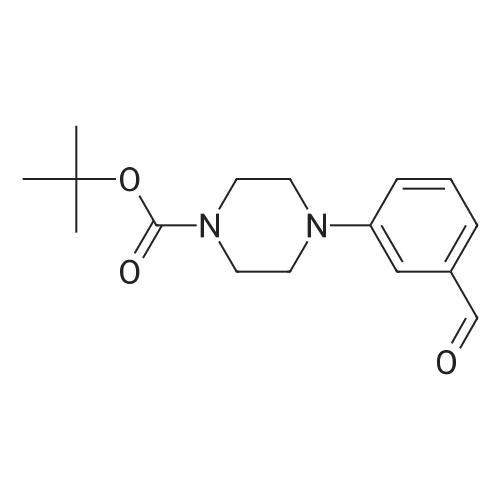
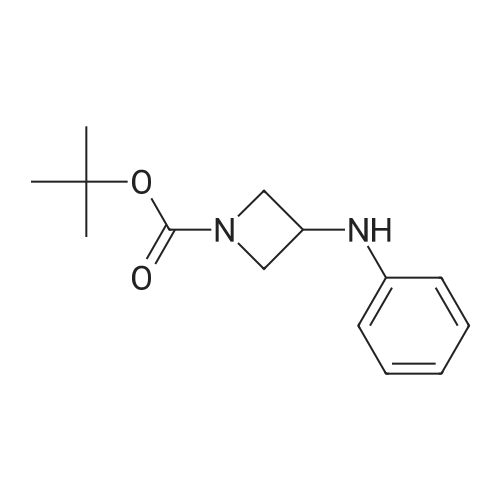
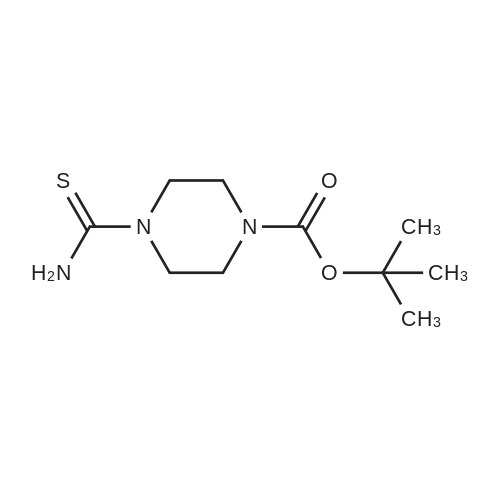
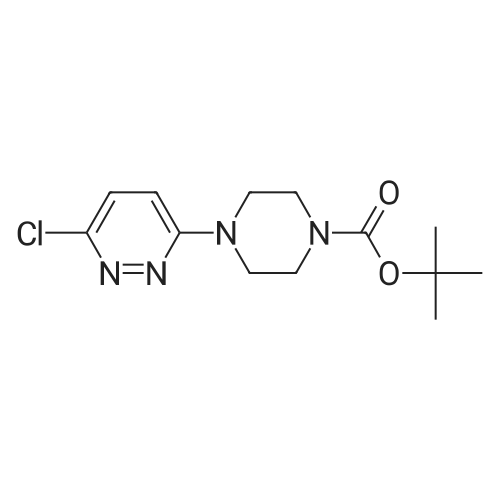
| 63% |
With triethylamine; In toluene; at 110℃; for 16.0h; |
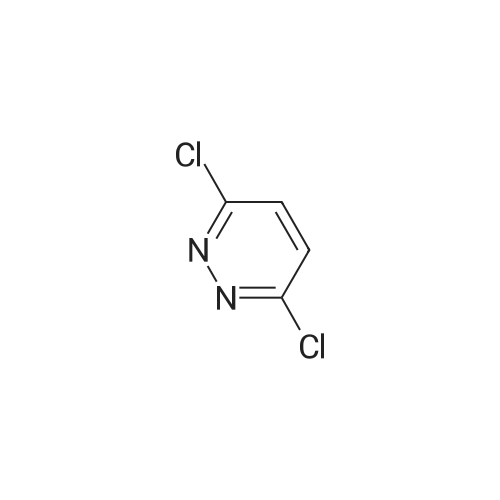
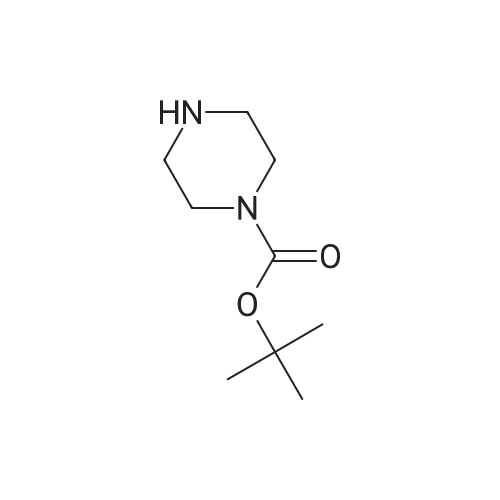
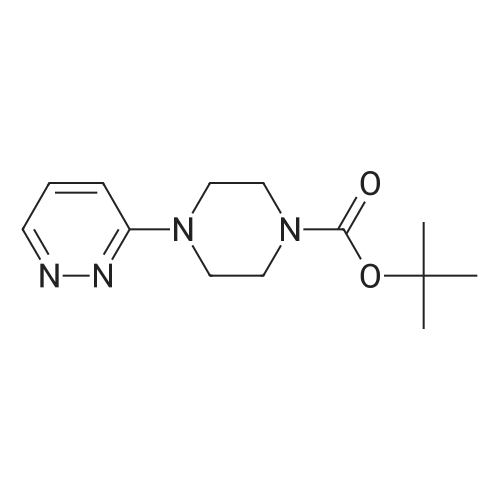
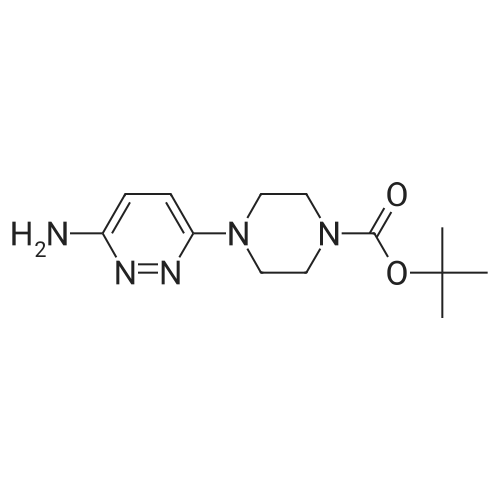
Preparation of tert-Butyl 4-(6-chloropyridazin-3-yl)piperazine-1-carboxylate 6-3: (1202) (1203) A stirred mixture of 3,6-dichloropyridazine 6-1 (2.0 g, 13.4 mmol), tert-butyl piperazine- 1-carboxylate 6-2 (3.72 g, 20.0 mmol) and triethylamine (2.78 mL, 20.0 mmol) in toluene (20mL) was heated at 110 C for 16 h. After complete consumption of 6-1 as evident from TLC, the volatiles were stripped off, residue partitioned between ethyl acetate and water, combined organic extracts evaporated to afford a crude residue which was purified column chromatography (elution with 30% ethyl acetate/Hexane) to afford tert-butyl 4-(6-chloropyridazin-3- yl)piperazine-1-carboxylate 6-3 (2.52 g, 8.46 mmol, 63.0 %) as an off-white solid. LC MS: ES+ 299.2 |
|
With triethylamine; In butan-1-ol; for 5.0h;Heating / reflux; |
A mixture of 13.52 g of tert-butyl 1-piperazinecarboxylate, 10.81 g of 3,6-dichloropyridazine and 20 ml of triethylamine in 100 ml of n-butanol is heated at reflux for 5 hours. It is concentrated under vacuum and the residue is chromatographed on silica gel, eluting with a DCM/AcOEt (90/10; v/v) mixture. This gives 14 g of the expected product, which is used as it is. |
|
With N-ethyl-N,N-diisopropylamine; In tetrahydrofuran; at 140℃; for 3.0h;Microwave irradiation; |
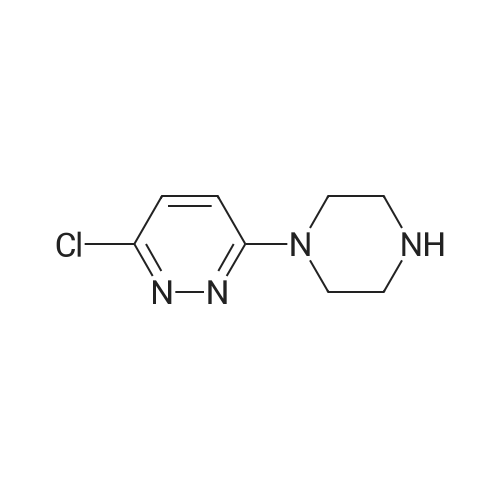
EXAMPLE 92; 5,5-Dimethyl-2-(6-[r6-piperazin-l-ylpyridazin-3-yl)oxy]-2,3-dihydro-4H-L4- benzoxazin-4-vU -5.6-dihydro- 1 ,3 -benzothiazol-7(4/f)-one; A mixture of 3,6-dichloropyridazine (0.60 g, 3.24 mmol), l-5(9C-piperazine (0.48 g, 3.24 mmol) and DIPEA (0.60 tnL, 3.24 mmol) in THF (20 mL) was heated to 14O0C under microwave irradiation for 3h. After cooling to r.t. it was concentrated in vacuo and purified by column chromatography (SiO2, 0-50% EtOAc in heptane) to give a white solid. A mixture of this material (crude pyridazinyl piperazine, 45 mg, 0.15 mmol),Example 6 (50 mg, 0.15 mmol) and cesium carbonate (98 mg, 0.3 mmol) in DMF (4 mL) was heated to 12O0C under microwave irradiation for 2h. After cooling to r.t. the mixture was concentrated in vacuo and purified by prep HPLC to give an off-white solid (26 mg, 30%). deltaH (CDCl3) 1.13 (6H, s), 1.48 (9H, s), 2.41 (2H, s), 2.74 (2H, s), 3.14 (8H, br.s), 4.15-4.24 (2H, m), 4.30-4.38 (2H5 m), 6.89-7.00 (2H, m), 7.26 (2H, s), 7.89 (IH, d, J 2.4 Hz). LCMS (ES+) 593 (M+H)+. |
|
With triethylamine; In butan-1-ol; for 5.0h;Heating / reflux; |
A mixture of 13.52 g of tert-butyl 1-piperazinecarboxylate, 10.81 g of 3,6-dichloro-pyridazine and 20 ml of triethylamine in 100 ml of n-butanol is refluxed for 5 hours. The mixture is concentrated under vacuum and the residue is chromatographed on silica gel, eluting with a DCM/EtOAc mixture (90/10; v/v). 14 g of the expected product are obtained, and are used without further purification. |
|
With N-ethyl-N,N-diisopropylamine; In tert-butyl alcohol; at 100 - 150℃; for 0.833333h;Microwave irradiation; |
Intermediate 141 ,1 -dimethylethyl 4-(6-chloro-3-pyridazinyl)-1 -piperazinecarboxylate In a microwave vial were mixed: 1 ,1 -dimethylethyl 1 -piperazinecarboxylate(135 mg, 0.725 mmol, available from Fluka), 3,6-dichloropyridazine (90 mg, 0.604 mmol, available from Alfa Aesar) and DIPEA (0.137 mL, 0.785 mmol) in Tert-Butanol (2 mL). The reaction was stirred and heated in an Emrys Optimizer microwave at 100C for 20 mins then for 30 mins at 150C. The reaction mixture was partitioned between EtOAc (20mL) and water (20mL) and the organic layer washed with brine (20mL) before being dried through an hydrophobic frit and concentrated. The residue was dissolved in DCM and purified by SP4 on a 12+M silica cartridge using a gradient of 10-50% EtOAc in cyclohexane. The appropriate fractions were collected and concentrated to yield the desired product as a white solid, 1 ,1 -dimethylethyl 4-(6-chloro-3-pyridazinyl)-1 - piperazinecarboxylate (1 14.2 mg). LCMS (Method C): Rt = 0.85, MH+ = 299 |
|
With N-ethyl-N,N-diisopropylamine; In tert-butyl alcohol; at 100 - 150℃; for 0.833333h;Microwave irradiation; |
In a microwave vial were mixed: 1,1-dimethylethyl 1-piperazinecarboxylate (135 mg, 0.725 mmol, available from Fluke), 3,6-dichloropyridazine (90 mg, 0.604 mmol, available from Alfa Aesar) and DIPEA (0.137 mL, 0.785 mmol) in Tert-Butanol (2 mL). The reaction was stirred and heated in an Emrys Optimizer microwave at 100 C. for 20 mins then for 30 mins at 150 C. The reaction mixture was partitioned between EtOAc (20 mL) and water (20 mL) and the organic layer washed with brine (20 mL) before being dried through an hydrophobic frit and concentrated. The residue was dissolved in DCM and purified by SP4 on a 12+M silica cartridge using a gradient of 10-50% EtOAc in cyclohexane. The appropriate fractions were collected and concentrated to yield the desired product as a white solid, 1,1-dimethylethyl 4-(6-chloro-3-pyridazinyl)-1-piperazinecarboxylate (114.2 mg). LCMS (Method C): Rt=0.85, MH+=299 |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping