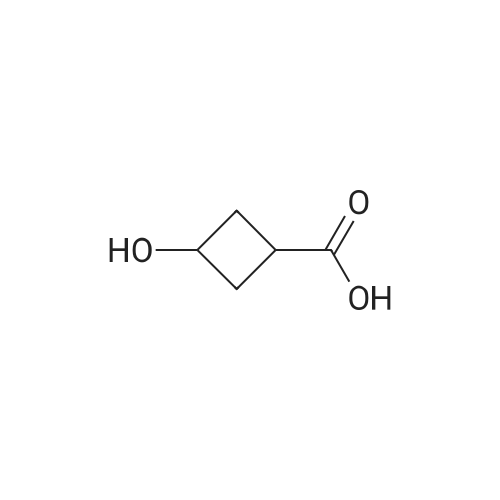
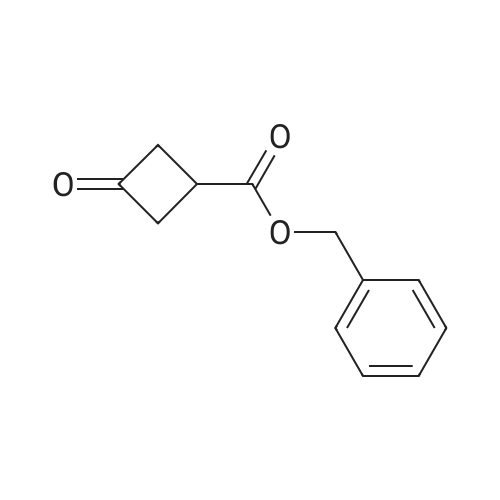
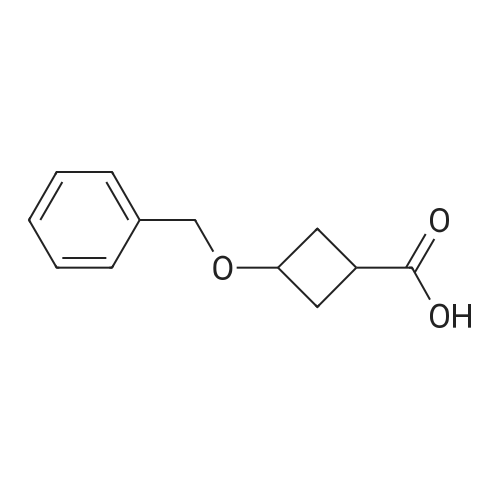
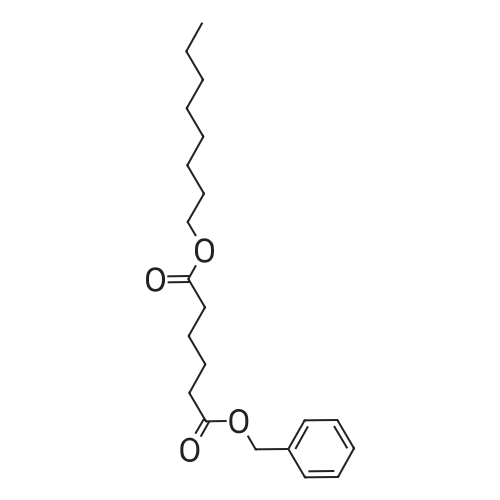
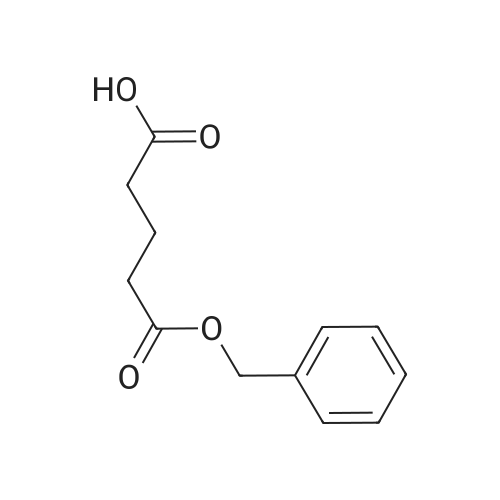
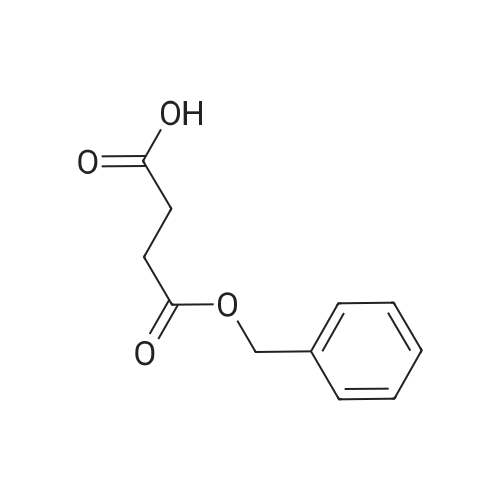
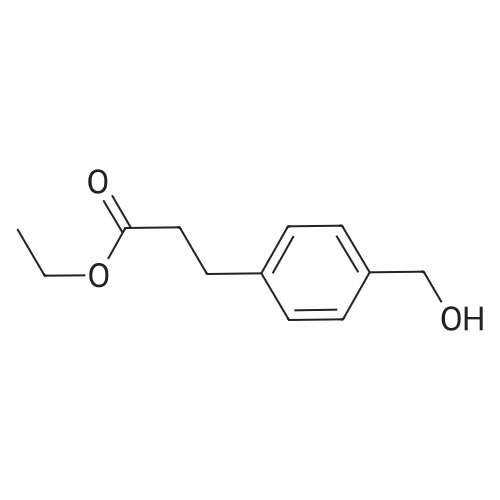
| 88% |
With sodium tetrahydroborate; water; In tetrahydrofuran; at 0 - 20℃; for 1h; |
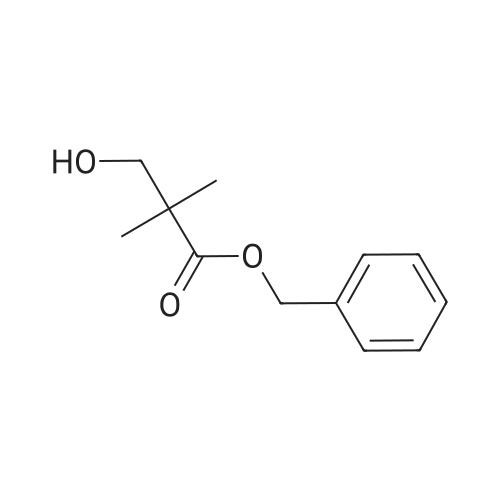
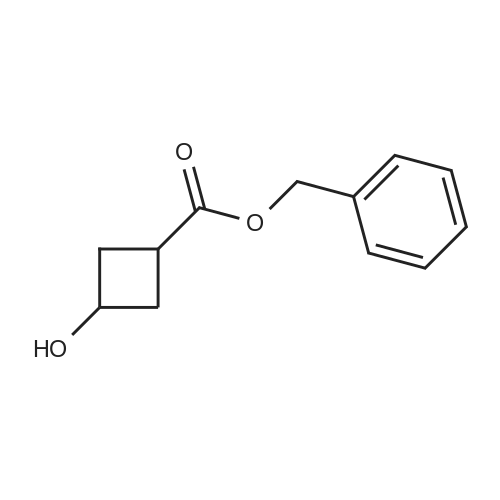
Example 261 : 2-N-Methyl-6-[5-(3-phenoxycyclobutyl)-1 ,2,4-oxadiazol-3-yl]-2-N-phenyl- 1 ,3,5-triazine-2,4-diamineTriethylamine (2 mL, 14.04 mmol) and benzyl bromide (1.2 mL, 10.0 mmol) were added to a solution of 3-oxocyclobutane-1-carboxylic acid (1.0 g, 8.77 mmol) in THF (10 mL) and the mixture was stirred at room temperature for 2 h. EtOAc (10 mL) was added and the mixture was washed with water followed by 1 M hydrochloric acid and then brine. The organic layer was dried over sodium sulfate and concentrated under vacuum. The residue was purified by FCC, eluting with a gradient of 0-15% EtOAc in hexane to afford <strong>[198995-91-4]benzyl 3-oxocyclobutane-1-carboxylate</strong> (0.938 g, 53%). A portion of benzyl 3- oxocyclobutane-1-carboxylate (0.800 g, 3.92 mmol) was dissolved in a mixture of THF (2.5 mL) and water (2.5 mL) and cooled to 0 C. Sodium borohydride (0.051 g, 1.96 mmol) was added and the mixture was stirred at room temperature for 1 h. The mixture was concentrated under vacuum and EtOAc (10 mL) was added. This was washed with water and then brine and the organic layer was then dried over sodium sulfate and concentrated. The residue was purified by FCC, eluting with a gradient of 0-10% EtOAc in hexane to afford benzyl 3-hydroxycyclobutane-1-carboxylate (0.715 g, 88%). A portion of benzyl 3-hydroxycyclobutane-1-carboxylate (0.400 g, 1.94 mmol) was dissolved in THF (10 mL) and phenol (0.547 g, 5.83 mmol) and triphenylphosphine (0.662 g, 2.52 mmol) were added. Diethyl azodicarboxylate (0.4 mL, 2.52 mmol) was added gradually and the mixture was stirred at room temperature for 24 h. The mixture was evaporated and then extracted with EtOAc (3 x 10 mL). The organic layer was washed with brine and concentrated under vacuum. The residue was purified by FCC, eluting with a gradient of0- 15% EtOAc in hexane to afford benzyl 3-phenoxycyclobutane-1-carboxylate (0.450 g, 82%). A portion of benzyl 3-phenoxycyclobutane-1-carboxylate (0.400 g, 1.42 mmol) was dissolved in EtOH (10 mL) and 10% palladium on carbon (0.010 g) was added. The mixture was stirred under an atmosphere of hydrogen at room temperature for 2 h. The mixture was filtered and the filtrate was evaporated. The residue was purified by FCC, eluting with a gradient of 0-10% EtOAc in hexane to afford 3-phenoxycyclobutane-1- carboxylic acid (0.245 g, 95%). 2-N-Methyl-6-[5-(3-phenoxycyclobutyl)-1 ,2,4-oxadiazol-3- yl]-2-N-phenyl-1 ,3,5-triazine-2,4-diamine was then prepared from 3-phenoxycyclobutane-1- carboxylic acid (0.190 g, 0.985 mmol) and 4-amino-N-hydroxy-6- [methyl(phenyl)amino]-1 ,3,5-triazine-2-carboximidamide (prepared in an analogous manner to Intermediate 1 , 0.150 g, 0.579 mmol) according to the method described for Example 209. The residue was purified by FCC, eluting with 10% MeOH in hexane to afford the title compound as a mixture of isomers (0.095 g, 42%). |
| 53% |
With methanol; sodium tetrahydroborate; In tetrahydrofuran; at 0℃; for 0.5h; |
Intermediate 32: benzyl 3-hydroxycyclobutanecarboxylate NaBH4 (215 mg; 5.68 mmol) was added to the solution of <strong>[198995-91-4]benzyl 3-oxocyclobutanecarboxylate</strong> (2.3 g; 11.3 mmol) in THF (30 mL) and MeOH (1.5 mL). The reaction mixture was stirred for 0.5 hour at 0 C., diluted with water (20 mL), and extracted with DCM (50 mL*2). The combined organic layers were dried over anhydrous Na2SO4, filtered, concentrated, and purified by reverse phase flash chromatography to afford 1.24 g (53%) of the title compound as a yellow oil. 1H NMR (400 MHz, CDCl3) delta [ppm]: 7.49-7.22 (m, 5H), 5.12 (d, J=4.1 Hz, 2H), 4.28-4.08 (m, 1H), 2.72-2.50 (m, 3H), 2.41-2.09 (m, 3H). |
| 47% |
With methanol; sodium tetrahydroborate; at 0 - 20℃; for 1h; |
To a solution of <strong>[198995-91-4]benzyl 3-oxocyclobutanecarboxylate</strong> (17.9 g, 88 mmol) in MeOH (150 ml) at 0C added sodium borohydride (3.32 g, 88 mmol) slowly. After addition, the reaction mixture was allowed slowly warm up to room temperature. After stirred for one hour, the reaction quenched with ice, extracted with 3x60 ml ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate and concentrated under vacuum. Then applied onto a silica gel column and eluted with ethylacetate/hexane 0-100%. This resulted in 8.5 g (47%) of benzyl 3hydroxycyclobutanecarboxylate as colorless oil. Then separated by ChiralPak AY-20um (300x20mmI.D). Mobile phase: A for SFC C02 and B for isopropanol, gradient 10% B. This result in trans-benzyl 3-hydroxycyclobutanecarboxylate (4g, Rt = 3.51 min). LC-MS (ES, m/z) C, 2H 1403: 206; Found: 207[M+H]+, cis-benzyl 3- hydroxycyclobutanecarboxylate (361 mg, 2.83 min). LC-MS (ES, m/z) Ci2Hi403: 206; Found: 207[M+H]+ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping