|
In ethanol; |
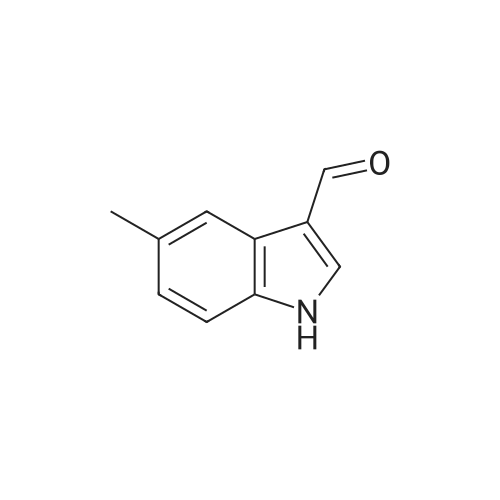
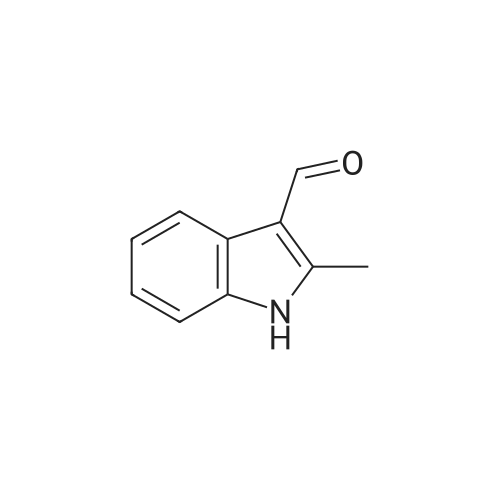
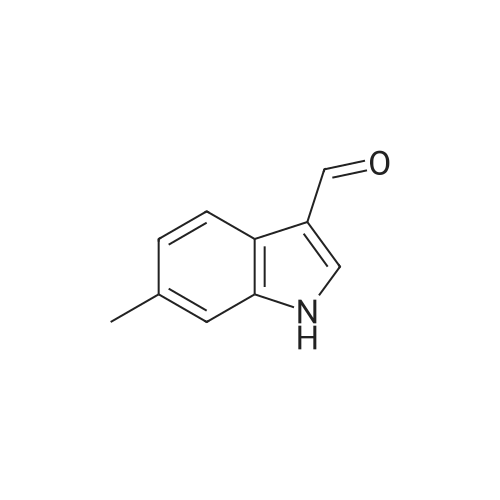
EXAMPLE 106 (Z)-2-(beta-D-Glucosylthio)-3-(6-methylindol-3-yl)-1-(3,4,5-trimethoxyphenyl)-2-propen-1-one (Compound 106) 2-(beta-D-Glucosylthio)-3',4',5'-trimethoxy-acetophenone (1.01 g) obtained in Reference Example 17 and <strong>[4771-49-7]6-methylindole-3-carbaldehyde</strong> (0.40 g) were dissolved in ethanol (10 ml), and piperidine (0.21 g) was added thereto, followed by heating under reflux for 24 hours. The reaction solution was concentrated under reduced pressure and the residue was purified by silica gel column chromatography. The obtained crude crystals were recrystallized from ethanol and purified by preparative HPLC (YMC pack ODS, SH-343-5, S-5, 120A, 250*20 mm, acetonitrile:water=40:60). The elude was concentrated under reduced pressure and the residue was recrystallized from a mixed solvent of ethanol and isopropyl ether (1:1) to give Compound 106 (322.2 mg). 1 H-NMR (270 MHz, DMSO-d6) delta2.40 (s, 3H), 2.78 (m, 1H), 3.16-3.32 (m, 5H), 3.79 (s, 3H), 3.80 (s, 3H), 3.81 (s, 3H), 4.24 (t, J=5.6 Hz, 1H), 4.76 (d, J=8.6 Hz, 1H), 4.82 (d, J=4.6 Hz, 1H), 5.09 (d, J=2.9 Hz, 1H), 5.46 (d, J=5.0 Hz, 1H), 6.94 (d, J=8.3 Hz, 1H), 7.09 (s, 2H), 7.26 (s, 1H), 7.33 (d, J=8.3 Hz, 1H), 7.52 (s, 1H), 8.12 (d, J=2.5 Hz, 1H), 11.73 (s, 1H) FAB-MS m/z=545 (M+ +1) Elemental Analysis: C27 H31 NO9 S.0.8H2 O Calcd.(percent): C, 57.91; H, 5.87; N, 2.50 Found (percent): C, 57.88; H, 5.77; N, 2.40 |
|
In ethanol; |
Example 14 (Z)-2-(beta-D-Glucosylthio)-3-(6-methylindol-3-yl)-1-(3,4,5-trimethoxyphenyl)-2-propen-1-one (Compound 14) 2-(beta-D-Glucosylthio)-3',4',5'-trimethoxyacetophenone (1.01 g) obtained in Reference Example 17 and <strong>[4771-49-7]6-methylindole-3-carbaldehyde</strong> (0.40 g) were dissolved in ethanol (10 ml), and piperidine (0.21 g) was added thereto, followed by heating under reflux for 24 hours. The reaction solution was concentrated under reduced pressure and the residue was purified by silica gel column chromatography. The obtained crude crystals were recrystallized from ethanol and purified by preparative HPLC (YMC pack ODS, SH-343-5, S-5, 120A, 250 x 20 mm, acetonitrile:water = 40:60). The elude was concentrated under reduced pressure and the residue was recrystallized from a mixed solvent of ethanol and isopropyl ether (1:1) to give Compound 14 (322.2 mg). 1H-NMR (270 MHz, DMSO-d6) delta 2.40 (s, 3H), 2.78 (m, 1H), 3.16-3.32 (m, 5H), 3.79 (s, 3H), 3.80 (s, 3H), 3.81 (s, 3H), 4.24 (t, J = 5.6 Hz, 1H), 4.76 (d, J = 8.6 Hz, 1H), 4.82 (d, J = 4.6 Hz, 1H), 5.09 (d, J = 2.9 Hz, 1H), 5.46 (d, J = 5.0 Hz, 1H), 6.94 (d, J = 8.3 Hz, 1H), 7.09 (s, 2H), 7.26 (s, 1H), 7.33 (d, J = 8.3 Hz, 1H), 7.52 (s, 1H), 8.12 (d, J = 2.5 Hz, 1H), 11.73 (s, 1H) FAB-MS m/z = 545 (M++1) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping