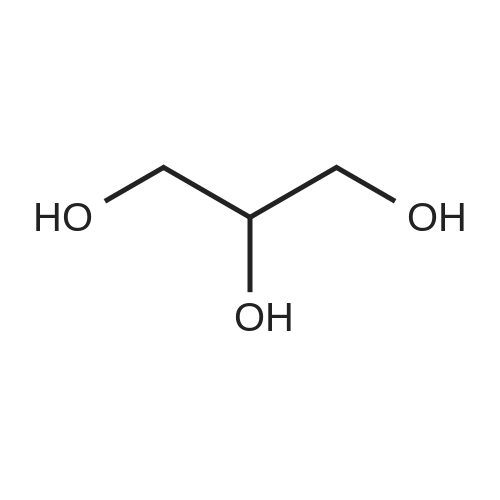
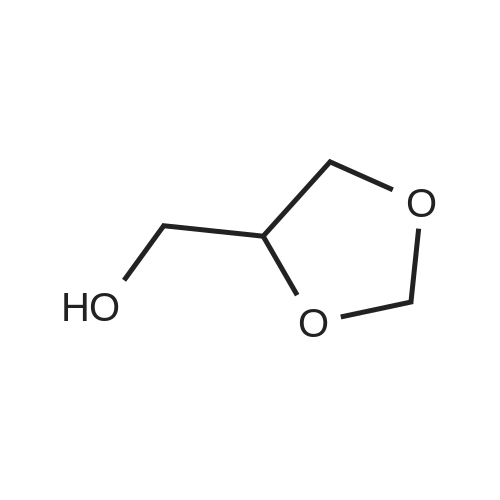
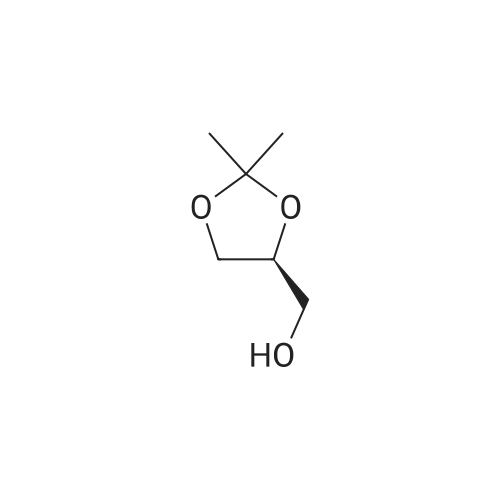
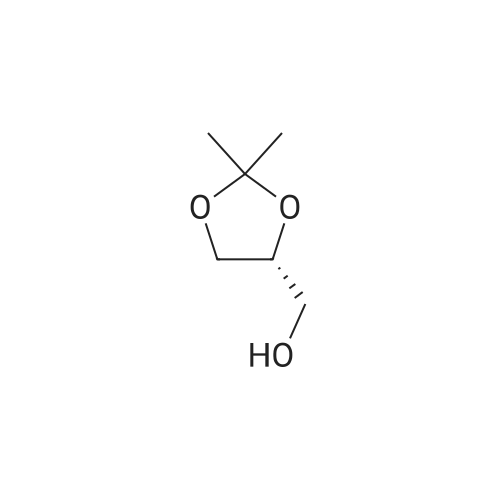
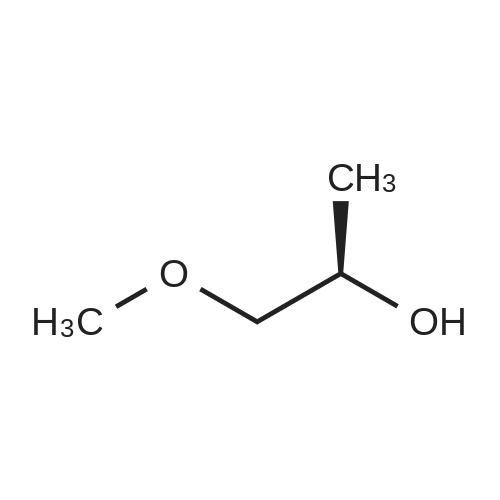
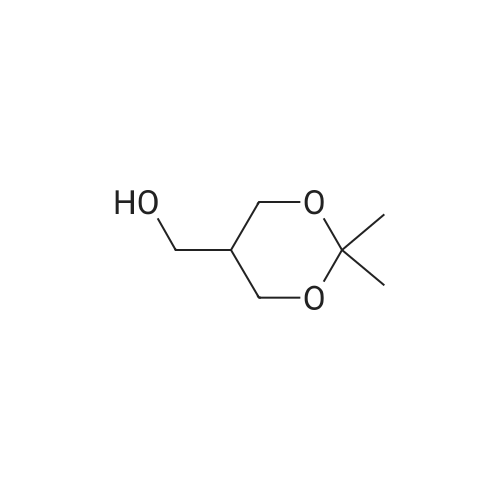
| 95.6% |
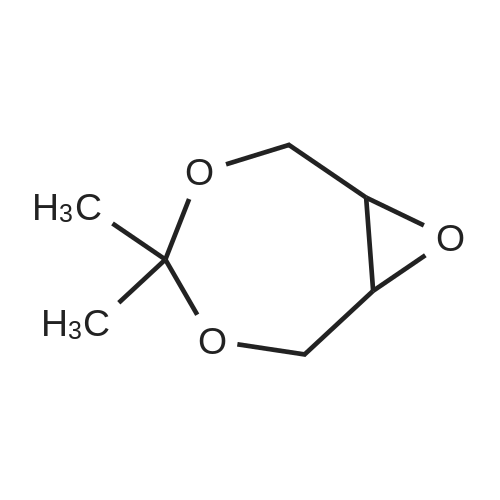
With dihydrogen peroxide; nitric acid; cetyltrimethylammonim bromide; ferric nitrate; In water; at 80℃; for 5.0h; |
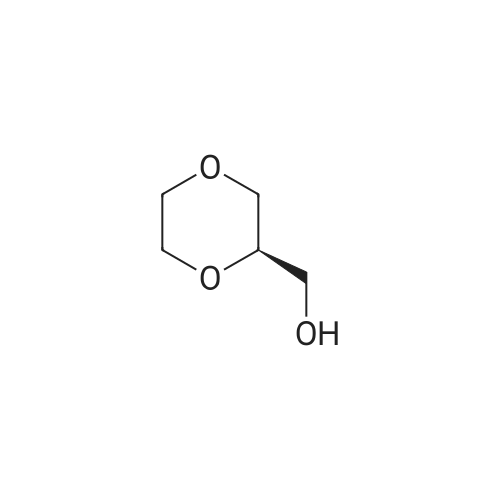
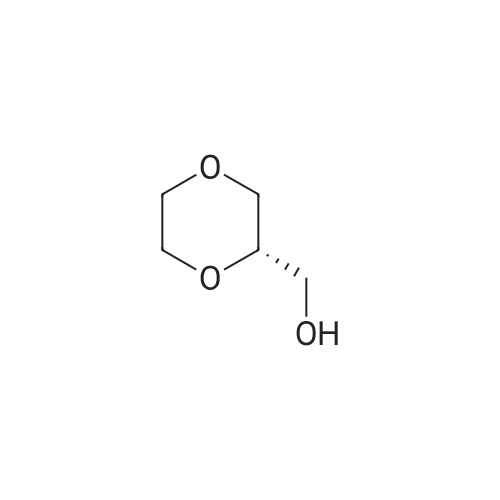
Will be 920g of glycerin,990gParaformaldehyde and20 g of the supported solid super acid catalyst prepared in Example 2 was addedIn the reaction device, the reaction is heated to 80 C, and the water having a lower boiling point and the trioxane formed in the reaction process enters the fractionation device.According to the principle that the boiling point of paraformaldehyde is lower than water, the triacetal is fractionated back to the reaction unit through a fractionation device.The reaction was carried out as a raw material, and the reaction was completed for 5 hours to obtain glycerol formal, and the product was analyzed by HPLC-MS.At 96.3%, the ratio of the six-membered ring product to the product of the five-membered ring in the product was 70:25. |
|
With Dowex 50; In toluene; at 100℃;Dean-Stark; Reflux; |
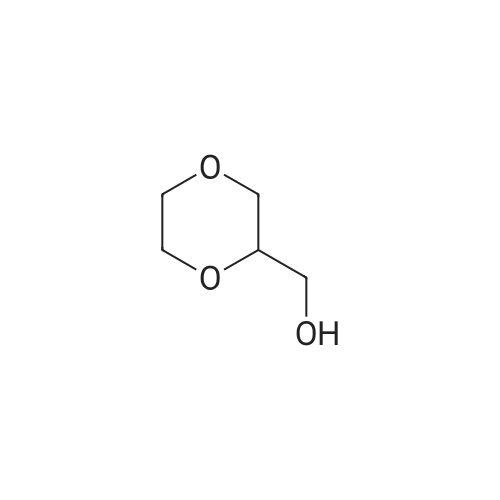
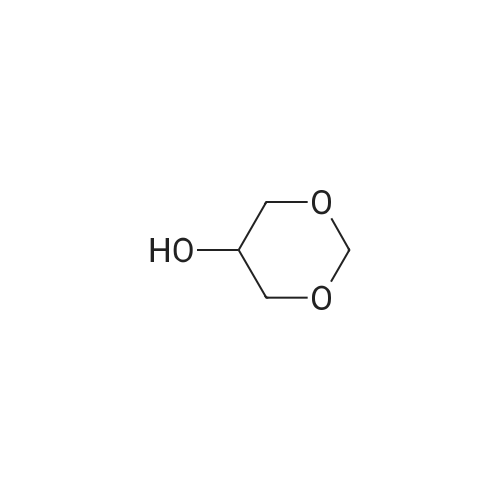
A 250 mL three neck flask equipped with a stirrer, an addition funnel, and a Dean-Stark trap was charged with glycerol (9.2 g, 0.1 mol), paraformaldehyde (2.7 g, 0.09 mol), activated ion exchange resin Dowex 50 (10 wt.%), and anhydrous toluene (70 mL). The reaction mixture was refluxed until calculated amount of water was distilled off. The mixture was cooled and filtered. The solvent was removed in low vacuum. Distillation of the residue afforded a mixture of compounds 1a and 1b in the yield of 9.2 g (93%), b.p. 190-195 C (760 Torr). An isomeric ratio was determined by 1H NMR spectroscopy from the integrated intensity ratio of the signals of H(4) methyne protons (deltaH 3.5-4.0) of dioxolane 1a and H(5) methyne proton (deltaH 3.5-4.0) of dioxane 1b. Compound 1a. MS (EI, 70 eV), m/z (Irel (%)): 103 [M - H]+ (not detected), 73 (75), 57 (15), 45 (100), 31 (15). Compound 1b. MS (EI, 70 eV), m/z (Irel (%)): 103 [M - H]+ (not detected), 87 (1), 74 (31), 45 (17), 44 (100), 31 (12). |
|
With 1,3,5-Trioxan; at 80℃; for 5.0h; |
920 g of glycerin, 990 g of trioxane and 20 g of the supported solid superacid catalyst prepared in Example 2 were charged into a reaction apparatus, and heated to 80 C to react, and the lower boiling water and trioxane formed during the reaction were fractionated. The device, according to the principle that the boiling point of the paraformaldehyde is lower than water, the triacetal is fractionated back to the reaction device through the fractionation device to continue the reaction as a raw material, and the reaction is finished for 5 hours, thereby obtaining glycerol formal, which is analyzed by HPLC-MS. The yield of the product was 93.5%, and the ratio of the product of the six-membered ring product to the product of the five-membered ring was 77:23. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping