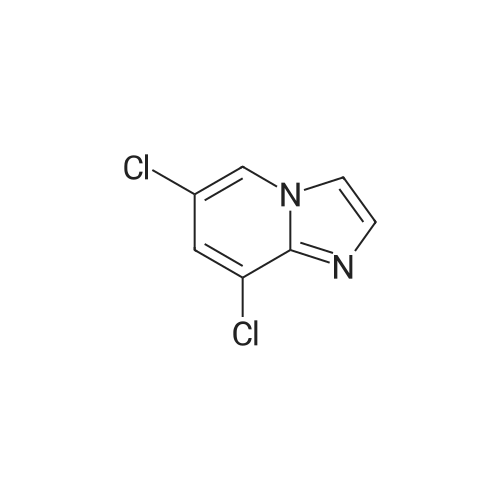
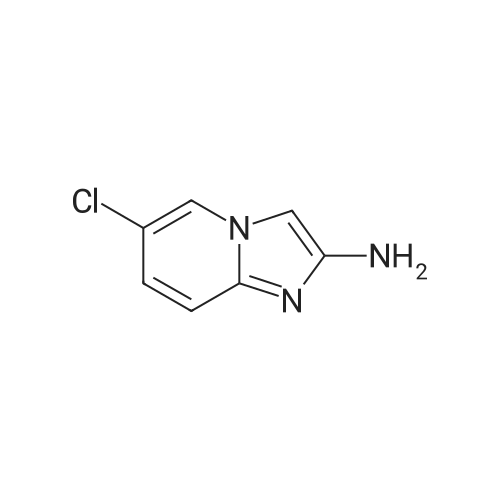
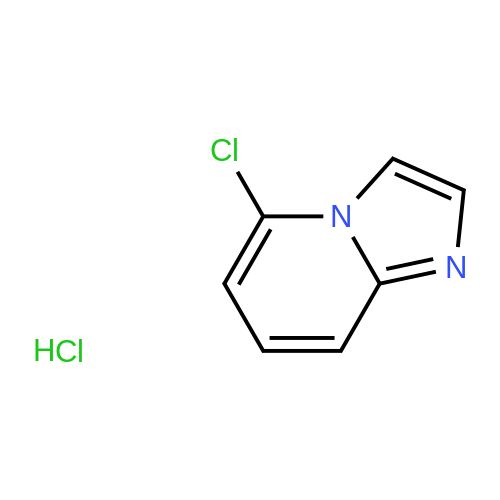
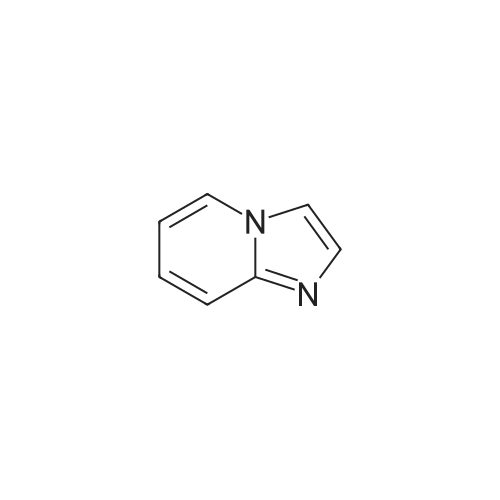
|
With sodium hydrogencarbonate; In ethanol; for 6h;Heating / reflux; |
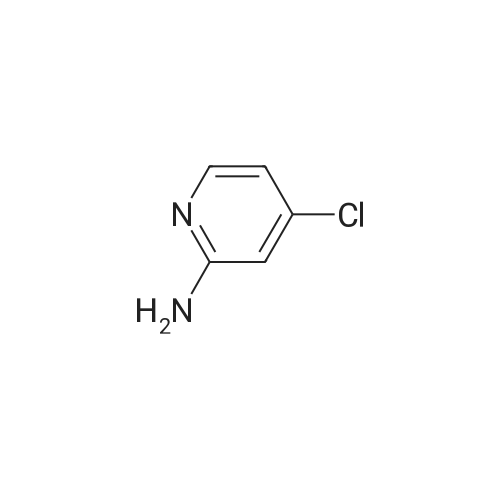
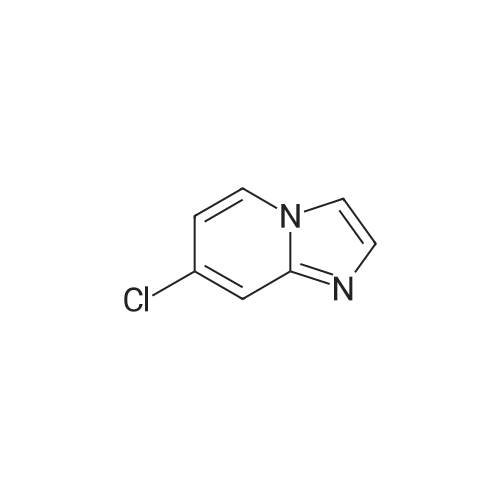
General Route A; Procedure A1 - General im idazopyridine ring formation; To a solution of 4-Chloro-pyridin-2-ylamine (12.8 g, 100 mmol, 1.0 equiv) in EtOH (170 ml) was added NaHCO3 (16.8 g, 200 mmol, 2.0 equiv) followed by chloroacetaldehyde (19.0 ml, 150 <n="95"/>mmol, 1.5 equiv). The mixture was refluxed for 6 h. Solvents removed under reduced pressure and the crude mixture was partitioned between water and EtOAc. The organic layer was washed with brine, dried (MgSO4), filtered and concentrated under reduced pressure. The product was purified by column chromatography (SiO2, eluted with 50% EtOAC-petrol) to afford 13.2 g of product. |
|
With sodium hydrogencarbonate; In ethanol; for 6h;Heating / reflux; |
To a solution of 4-Chloro-pyridin-2-ylamine (12.8 g, 100 mmol, 1.0 equiv) in EtOH (170 ml) was added NaHCO3 (16.8 g, 200 mmol, 2.0 equiv) followed by chloroacetaldehyde (19.0 ml, 150 mmol, 1.5 equiv). The mixture was refluxed <n="112"/>for 6 h. Solvents removed under reduced pressure and the crude mixture was partitioned between water and EtOAc. The organic layer was washed with brine, dried (MgSO4) and concentrated under reduced pressure. The product was purified by column chromatography (SiO2, eluted with 50% EtOAC-petrol) to afford 13.2 g of product. MS: [M+H]+ 153 |
|
With sodium hydrogencarbonate; In ethanol; for 17h;Heating / reflux; |
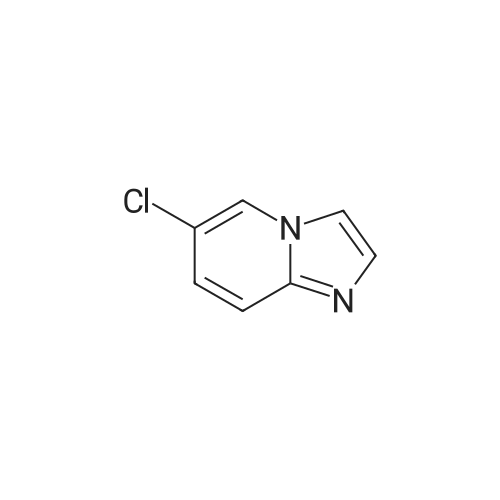
4-Chloro-pyridin-2-ylamine (1 eq, 38.9 mmol, 5 g) is added to a solution of chloroacetic aldehyde (3 eq, 117 mmol, 15.1 ml) in EtOH (60 ml). NaHCO3 (2 eq, 77.8 mmol, 6.53 g) is added and the reaction mixture is heated at reflux for 17 h. The solvent is removed in vacuo and the product is purified by flash column chromatography eluting with 8:2 DCM/MeOH to afford 7-chloro-imidazo- [1,2-a]- pyridine as a red solid; [M+H]+ = 153 |
|
With sodium hydrogencarbonate; In ethanol; water; for 10h;Reflux; |
To a mixture of 4-chloropyridin-2-amine (1.0 g, 7.78 mmol, 1.0 eq) and NaHCCb (1.31 g, 15.56 mmol, 2.0 eq) in EtOH (18 mL) was added chloroacetaldehyde, 50% wt in water, (1.48 mL, 11.67 mmol, 1.5 eq). The reaction mixture was heated to reflux. After 10 h, the solvent was removed under reduced pressure and the residue was partitioned between EtOAc: H20 (1 : 1, 100 mL). The organic layer was washed with Brine (50 mL), dried (MgS04), filtered and concentrated. The material was taken through without further purification. (0243) LCMS: RT = 0.123 min, >98% 215 and 254 nM, m/z = 153.0 [M + H]+. |
|
With sodium hydrogencarbonate; In ethanol; for 6h;Reflux; |
To a solution of 4-Chloro-pyridin-2-ylamine (12.8 g, 100 mmol, 1.0 equiv) in EtOH (170 ml) was added NaHCO3 (16.8 g, 200 mmol, 2.0 equiv) followed by chloroacetaldehyde (19.0 ml, 150 mmol, 1.5 equiv). The mixture was refluxed for 6 h. Solvents removed under reduced pressure and the crude mixture was partitioned between water and EtOAc. The organic layer was washed with brine, dried (MgSO4), filtered and concentrated under reduced pressure. The product was purified by column chromatography (SiO2, eluted with 50% EtOAC-petrol) to afford 13.2 g of product. MS: [M+H]+ = 153. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping